Safety of enteral ventilation “buttock breathing” demonstrated in a first-in-human trial
A major step forward toward clinical application to patients with severe respiratory failure
- For the first time in the world, safety and tolerability of intrarectal perfluorodecalin (PFD), a liquid oxygen carrier, for enteral ventilation in a clinical trial involving healthy adults.
- As a result of gradually administering the drug from 25 mL to 1500 mL, no serious side effects were observed, and mild gastrointestinal symptoms, such as abdominal bloating and pain were transient and recovered spontaneously.
- Post-administration blood tests showed no detection of PFD, confirming that it is not absorbed into the body.
- This result marks a major step toward clinical application of enteral ventilation, which was currently at the animal testing stage. It is expected that clinical tests using oxygen-rich fluids will lead to the development of new treatments for severe respiratory failure.
Outlines
A research team including Professor Takanori Takebe of the Human Biology Research Unit at the Institute of Integrated Research at the Science Tokyo (also holds multiple positions as Professor of Graduate School of Medicine and Vice Director of the Premium Research Institute for Human Metaverse Medicine of the University of Osaka), and Associate Professor of Hospital Tasuku Fujii of the Department of Anesthesiology, Nagoya University Hospital, has demonstrated through the first-in-human trial that a single intrarectal dose of perfluorodecalin (PFD), a liquid used in enteral ventilation, is safe and well tolerated in humans.
This research was conducted in 27 healthy adult males aged 20 to 45 years, with the aim of evaluating the safety and tolerability of gradually increasing administration of non-oxygenated PFD. The results did not reveal any serious adverse events or dose-limiting toxicities. Mild gastrointestinal symptoms, such as abdominal bloating and pain were observed in the high-dose group, but these were transient and recovered spontaneously without the need for special treatment.
Furthermore, post-administration blood tests revealed no abnormalities in any items, including liver and kidney function, and no PFD was detected in the blood. These results suggest that PFD functions safely in the intestine without being absorbed into the body.
The achievement of this research paves the way for the clinical application of the innovative medical concept of "buttock breathing," which has currently remained at the animal testing stage, to humans. Based on the safety foundation established in this research, it will be possible to proceed to verify the therapeutic effects of oxygen-rich PFDs in patients with severe respiratory failure.
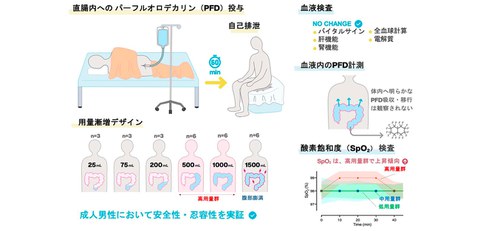
Fig. 1 Clinical trial design and results summary. A dose-escalation trial was conducted on healthy adult males using a method known as a 3+3 design, in which the PFD adiministration was gradually increased while safety was confirmed
Credit: Takanori Takebe
Research Background
Severe respiratory failure as represented by acute respiratory distress syndrome (ARDS), is a highly fatal condition in which the gas exchange function of the lungs is lost. Currently, ventilators and extracorporeal membrane oxygenation (ECMO) are used as standard treatments, but these face challenges as they place additional strain on the lungs and carry the risk of complications. For this reason, it has been expected in the world to develop an entirely new treatment that can supply oxygen to the entire body while allowing the lungs to rest.
The research team has previously reported the research results by using animal models that it is possible to mimic the intestinal breathing ability of some aquatic organisms and to supply oxygen to mammals through the intestines using enteral ventilation. However, before this innovative treatment could be applied to humans, a critical step was to first strictly evaluate its safety and tolerability in healthy human adults.
Research Contents
This was a phase 1, single-site, open-label, non-controlled, dose-escalation trial in 27 healthy adult males aged 20–45 years conducted in Japan. Participants received a single intrarectal dose of non-oxygenated perfluorodecalin (escalating from 25 mL to 1,500 mL) retained for 60 min.
As a result, the following points were clarified:
1. High safety: During the study period, no serious adverse events or side effects leading to treatment discontinuation occurred. Blood tests also showed no clinically significant changes in liver function (AST, ALT), kidney function, electrolytes, or any other items, confirming the safety of this therapy on organs.
2. High tolerability: Minor adverse events such as abdominal bloating and pain, and the urge to defecate were reported, but although these tended to occur more frequently in the high-dose group, they were all grade 1 (mild), transient, and recovered spontaneously. Doses up to 1000 mL were particularly well tolerated.
3. Non-absorbable into the body: Blood samples were taken over a 12-hour period following administration, and PFD concentrations were below the detection limit (1.0 μg/mL) in all blood samples. This is an important result indicating that PFD is not absorbed into the body from the intestinal tract and therefore poses an extremely low risk of causing systemic toxicity.
4. Possibility of oxygenation: The primary purpose of this study was to confirm safety, and a PFD that had not been actively provided with oxygen was used. However, in the high-dose group of 500 mL or more, a slight increase of approximately 1% in peripheral oxygen saturation (SpO₂) was observed. This suggests that oxygen from the atmosphere may be dissolved in the PFD, and the difference in concentration with venous blood may be what supplies oxygen to the body, supporting the principles of this method.
Social Impact of the Research
This important safety milestone paves the way for future development of an entirely new medical modality of enteral ventilation. By demonstrating safety and tolerability in a world’s first-in-human trial, this technology, which had previously remained at the basic research stage, has greatly opened up the possibility of reaching patients as an actual medical treatment.
Based on this solid evidence of safety, the research team will be able to proceed to the next step, clinical trials, to verify the therapeutic effects in actual patients. In the future, it is expected that this new medical technology will be established as a minimally invasive respiratory support treatment that can complement or replace ventilators and ECMO.
Future Development
Based on the safety data established in this study, the research team is now preparing for clinical trials using oxygen-enriched PFDs. If its effectiveness is proven, this revolutionary oxygenation therapy, which does not rely on lung function, could become a new means of saving patients with severe respiratory failure who have limited treatment options, including newborns.
Notes
The article, “Safety and Tolerability of Intrarectal Perfluorodecalin for Enteral Ventilation in a First-in-Human Trial,” was published in Med at DOI: 10.1016/j.medj.2025.100887 .
