Efficacy and safety of anamorelin for gastric cancer cachexia
The world's first randomized controlled trial for gastric cancer cachexia
- A randomized controlled trial for gastric cancer cachexia was conducted (total of ten institutions, 203 patients), demonstrating the efficacy and safety of anamorelin for gastric cancer cachexia.
- Cancer cachexia, characterized by weight loss and decreased skeletal muscle mass, has had few effective drug treatments. This study demonstrated that the anamorelin administration group had an increase in lean body mass and a significant increase in total body weight, showing the potential for anamorelin to become a novel treatment.
- In the anamorelin administration group, in addition to a significant improvement in appetite and diet-related QOL, the incidence rate of chemotherapy-related adverse events (loss of appetite, nausea) was also significantly reduced.
- The development of multifaceted approaches that combine drug therapy, nutritional therapy, and exercise therapy, as well as the provision of new cancer treatment options are expected.
Outlines
A research group including Associate Professor (Lecturer) Kazuyoshi Yamamoto (at the time of the research), Associate Professor Yukinori Kurokawa, and Professor Yuichiro Doki of the Department of Gastroenterological Surgery, Graduate School of Medicine, the University of Osaka, conducted a randomized controlled trial involving a total of ten facilities, including the graduate school and affiliated hospitals, and showed the efficacy and safety of anamorelin for treating gastric cancer cachexia.
Cancer cachexia is reported to occur in 50% to 80% of patients with advanced cancer, and is a serious complication that is particularly seen in lung cancer and gastrointestinal cancer. It is characterized by weight loss and a decrease in skeletal muscle mass, and the progression of metabolic abnormalities and catabolic reactions poses clinical challenges that make it difficult to continue treatment, and there are few effective drug treatments to date.
In this study, 203 patients who were planning to or were undergoing first- to third-line chemotherapy for unresectable advanced or recurrent gastric cancer with cachexia in addition to standard treatment were randomly assigned to either a group with anamorelin administration for 12 weeks (104 patients) or a group without anamorelin administration for the same period (99 patients). Ultimately, 101 patients in the anamorelin administration group and 97 patients in the non-anamorelin administration group were analyzed, and the primary endpoint was the change in lean body mass after eight weeks.
The results demonstrated that the anamorelin administration group showed increased body weight and appetite, and lean body mass tended to increase (+0.99 vs +0.14, P=0.063), with a significant difference observed in the analysis of covariance (ANCOVA) (+1.077 kg, P=0.018). No differences were observed in grip strength or chemotherapy response rate, but the incidence rate of chemotherapy-related side effects such as loss of appetite and nausea was reduced, and safety was also confirmed.
These results suggest that anamorelin may improve the nutritional status and patients’ QOL with advanced or recurrent gastric cancer with cachexia.
Fig. 1 Trial scheme
Credit: Yuichiro Doki
Research Background
Cancer cachexia is reported to occur in 50% to 80% of patients with advanced cancer, and is a serious complication that is particularly seen in lung cancer and gastrointestinal cancer. It is characterized by persistent weight loss and decreased skeletal muscle mass that cannot be reversed by nutritional supplementation alone, resulting in significant impairment of physical function and poor prognosis.
The complex interplay between tumor-derived factors and host inflammatory responses leads to the progression of metabolic abnormalities and catabolic reactions, making it difficult to continue treatment, posing a clinical challenge. However, to date, there have been few effective drug treatments, leaving significant unmet medical needs.
Against this background, it has been attracting attention on the gastrointestinal hormone ghrelin, which was discovered in 1999 by Japanese researcher Dr. Kenji Kangawa (Honorary Director of the National Cerebral and Cardiovascular Center) and his research group. Ghrelin has a variety of physiological effects, including appetite stimulation, weight gain, muscle mass increase, and anti-inflammatory effects. Furthermore, Professor Yuichiro Doki at the Graduate School of Medicine of the University of Osaka was the first in the world to report that blood ghrelin levels decrease significantly after gastrectomy, demonstrating the clinical importance of ghrelin. Since then, Dr. Kangawa and Professor Doki have conducted numerous collaborative research on the relationship between ghrelin and digestive cancer, and their findings have been highly praised in the fields of nutrition and oncology.
Anamorelin is an oral drug that mimics the effects of ghrelin, and in an international Phase III clinical trial (ROMANA 1/2) targeting non-small cell lung cancer-associated cancer cachexia, it was reported to improve appetite, body weight, and lean body mass (LBM). However, the efficacy of anamorelin in gastrointestinal cancers, particularly gastric cancer, has been limited to small-scale Phase III trials and observational studies, with little scientific evidence and its effectiveness is still an open question.
Research Contents
The research group conducted the world's first multicenter randomized controlled trial in patients with unresectable or recurrent gastric cancer to verify the efficacy and safety of anamorelin. A total of ten institutions participated in the trial, including the University of Osaka’s Graduate School of Medicine and affiliated hospitals.
In this study, 203 patients who were undergoing or planning to undergo first- to third-line chemotherapy for unresectable advanced or recurrent gastric cancer with cachexia were randomly assigned to receive 12 weeks of anamorelin administration (104 patients) or no anamorelin administration (99 patients). Ultimately, 101 patients in the anamorelin administration group and 97 patients in the non-anamorelin administration group were analized.
As a result, the primary endpoint, the change in lean body mass after eight weeks, showed a tendency for an increase in the anamorelin administration group compared to the non-administration group (+0.99 vs +0.14, P=0.063), and a significant difference was observed in in the analysis of covariance (ANCOVA) (+1.077 kg, P=0.018). The effect of increasing lean body mass by approximately one kilogram was equivalent to the results of the ROMANA-1 and -2 trials targeting non-small cell lung cancer-associated cancer cachexia, confirming the reproducibility of anamorelin's effect on lean body mass.
Additionally, the anamorelin administration group indicated an increase in total body weight and fat mass after eight weeks but no difference was observed in the grip strength.
No difference was observed in the efficacy rate of chemotherapy, but the incidence rate of chemotherapy-related side effects such as loss of appetite and nausea decreased, and there was a significant improvement in the diet-related QOL questions "Did you have an appetite?" and "Did you think the meal was tasty?".
Regarding safety, no serious adverse events were observed with anamorelin administration, except for one case of Grade 3 hyperglycemia.
The results of the subgroup analysis revealed that improvement in lean body mass was more likely to be observed in patients with ECOG PS 0–1 and relatively well-maintained physical strength, and in patients undergoing first-line chemotherapy with a short history of chemotherapy. These results suggest that anamorelin may improve the nutritional status and patients’ QOL with advanced or recurrent gastric cancer with cachexia.
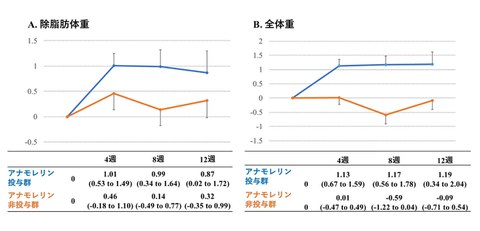
Fig. 2 Changes in lean body mass (left), and total body weight (right)
Credit: Yuichiro Doki
Outline of the randomized controlled trial
Subject: Patients with unresectable/advanced recurrent gastric cancer accompanied by cachexia who are planning to undergo or are undergoing first to third line chemotherapy
Period: November 17, 2021 - July 4, 2024
Intervention: Anamorelin administration group received 100 mg/day of anamorelin for 12 weeks
Primary endpoint: Change in lean body mass after eight weeks
Secondary endpoints: Body weight, fat mass, grip strength, nutritional index, QOL, chemotherapy-related side effects, safety, subgroup analysis
Primary endpoint (change in lean body mass after eight weeks)
Anamorelin administration group: +0.99 (+0.34 to +1.64) kg
Anamorelin non-administration group: +0.14 (-0.49 to +0.77) kg
Unadjusted between-group difference (95% confidence interval): +0.85 (-0.05 to 1.75) kg (P=0.063)
Adjusted between-group difference (95% confidence interval): +1.077(0.184 to 1.969) kg (P=0.018)
Although there was no significant difference before adjustments, a significant difference was observed in the analysis of covariance (ANCOVA).
Secondary endpoints
Change in total body weight after eight weeks: +1.17 (+0.56 to 1.78) vs. -0.59(-1.22 to +0.04) kg (P<0.0001)
Change in fat mass after eight weeks: +0.15 (-0.40 to +0.70) vs. -0.73 (-1.37 to -0.08) kg (P=0.040)
Change in grip strength after eight weeks: -0.13 (-1.04 to +0.78) vs. -0.65 (-1.41 to 0.12) kg (P=0.39)
Diet-related QOL: There was a significant improvement in the questions “Did you have an appetite?" and "Did you think the meal was tasty?"
Chemotherapy-related side effects: There was a significant reduction in Grade 2 or higher anorexia (10.6% vs. 23.2%, P=0.022) and nausea (0% vs. 6.3%, P=0.013)
Chemotherapy response rate: The objective response rate in first-line therapy was 31.7% versus 28.1%; in second-line therapy, 12.5% versus 21.1%; and in third-line therapy, 14.3% versus 0%. No statistically significant differences were observed between the two groups at any treatment line
Safety: There were no serious adverse events other than hyperglycemia (Grade 3, one patient). Safety was well confirmed
Fig. 3 Subgroup analysis
The improvement in lean body mass was more pronounced in patients with ECOG PS 0–1and those undergoing first-line chemotherapy
Credit: Yuichiro Doki
Social Impact of the Research
The results of this study showed that anamorelin may improve weight and appetite in patients with gastric cancer cachexia, helping them continue chemotherapy. This is an achievement that directly leads to an improvement in the patients’ QOL and has great social significance. Furthermore, the result of this study, based on the findings of ghrelin research, is expected to lead to the development of multifaceted approaches that combine drug therapy, nutritional therapy, and exercise therapy, providing new treatment options for cancers.
Notes
The article, “Efficacy and safety of anamorelin for cancer cachexia in patients with unresectable or recurrent gastric cancer: a multicentre, open-label, randomised controlled trial,” was published in British scientific journal of eClinicalMedicine (online) at DOI: 10.1016/j.eclinm.2025.103500.
