Developed a new technology to enhance the safety of antisense oligonucleotides (ASOs) drugs for treating neurological disorders
Potential application to a wide range of central nervous system diseases, including Alzheimer's disease
- A new technology that significantly improves safety while maintaining efficacy in antisense oligonucleotide drugs (ASOs) used to treat neurological disorders such as Alzheimer's disease has been developed.
- In experiments using mouse/human neuronal cells and mouse/rat models, a mechanism for improving delayed neurotoxicity, a challenge in drug development, was discovered.
- This technology may potentially alleviate restrictions on the dosage of ASOs, accelerating the development of therapeutic drugs for a wide range of central nervous system disorders, including Alzheimer's disease.
Outlines
Antisense oligonucleotides (ASOs) drugs primarily control the function of RNA, and are attracting attention as new therapeutic candidates for many neurological disorders, including Alzheimer's disease and amyotrophic lateral sclerosis (ALS). However, the problem is that late-onset and serious neurological side effects (central neurotoxicity) occur after intrathecal administration.
A research group including Project Professor Takanori Yokota, Project Junior Associate Professor Kotaro Yoshioka, Project Researcher Su Su Lei Mon, and Takayuki Kuroda (graduate student) of the Department of Neurology and Neurological Science and Nucleotide and Peptide Drug Discovery Center, Graduate School of Medical and Dental Sciences at Institute of Science Tokyo, in collaboration with a research group led by Professor Satoshi Obika and Associate Professor (Lecturer) Takao Yamaguchi of the Department of Bioorganic Chemistry, Graduate School of Pharmaceutical Sciences, the University of Osaka, demonstrated through experiments using neurons and mice/rats that by incorporating a new artificial nucleic acid, 5'-cyclopropylene (5'-CP), into ASOs, it is possible to significantly reduce nervous system side effects while maintaining efficacy.
Furthermore, the researchers investigated the mechanism of side effect reduction and discovered that abnormal localization of paraspeckle proteins within nerve cells is involved.
The safety of ASOs is improved by this technology and is expected to develop ASOs for a wider range of neurological disorders in the future.
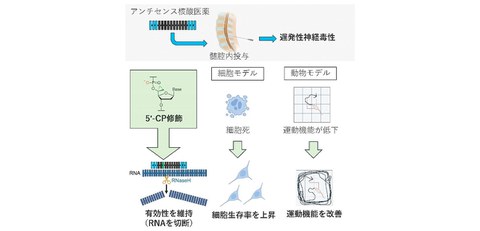
Fig. 1 Overview of this research
Credit: Satoshi Obika
Research Background
ASOs are a new type of drug that primarily regulates RNA function and modulates gene expression that is the cause of disease. In Japan, tofersen has been approved as a treatment for patients with amyotrophic lateral sclerosis (ALS), a debilitating neurological disease, who have mutations in the SOD1 gene, and is attracting a great deal of attention as a next-generation treatment.
However, intrathecal administration of ASOs may cause neurotoxicity, which is a major challenge in drug development. In particular, late-onset neurotoxicity (severe lower limb paralysis and sensory impairment) that appears several days to several weeks after administration has been reported in actual patients and is a critical issue that must be overcome.
Previous studies have suggested that one of the causes of ASO toxicity is abnormal interactions between ASOs and proteins [Reference 1]. Furthermore, it has been reported that by introducing artificial nucleic acids into the appropriate sites of ASOs, it may be possible to control this interaction and reduce side effects.
However, such technologies have not been fully examined for ASOs targeting the central nervous system.
Therefore, in this study, the research group introduced a new artificial nucleic acid, 5'-CP, developed by Professor Obika and his research team at the Graduate School of Pharmaceutical Sciences, the University of Osaka, into ASOs to examine whether it could reduce the late-onset neurotoxicity caused by ASOs.
Research Contents
First, the researchers examined the cytotoxicity of various ASOs when they were transfected into neurons.
Furthermore, they injected these ASOs intracerebroventricularly or intrathecally to mice and rats, and evaluated late-onset neurotoxicity in detail. Based on these results, the researchers established cell models and rodent models (mice and rats) that can reproduce the late-onset neurotoxicity caused by ASOs.
Next, through verification using these models, the research group discovered that introducing an artificial nucleic acid called 5'-CP into a specific position in the ASO could reduce late-onset neurotoxicity while maintaining efficacy.
Fig. 2 5'-CP ASO enhances the safety of ASO while maintaining efficacy
Furthermore, the researchers confirmed that this toxicity is caused by the abnormal localization of paraspeckle proteins in the nucleoli rather than in their natural location within neurons. They also demonstrated that the abnormal localization was normalized by introducing 5'-CP.
Credit: Satoshi Obika
Fig. 3 5'-CP ASO reduces ASO-induced abnormal localization of paraspeckle proteins (◀)
Fig. 4 Mechanisms of ASO-induced cytotoxicity and 5'-CP -mediated amelioration of toxicity
When the administered ASO (shown by the red line) interacts with paraspeckle proteins, it is abnormally localized in the nucleolus, causing stress and resulting in apoptosis. On the other hand, ASOs containing
5'-CP (shown in green) modulate their interaction with paraspeckle proteins, reducing their abnormal localization to the nucleolus.
Credit: Satoshi Obika
Social Impact of the Research
ASOs are expected to be a new therapeutic drug for many intractable neurological disorders for which no fundamental treatment exists. However, there is a trade-off between increasing the dosage to achieve high efficacy and side effects, and there have been cases where clinical trials have been suspended for some diseases. Therefore, avoiding side effects has been a major challenge.
This study demonstrated the possibility of avoiding the neurological side effects of ASOs while maintaining their efficacy. This will ease the dosage restrictions for ASOs and is expected to significantly advance the development of new therapeutic drugs using ASOs for a variety of neurological disorders that have been difficult to treat until now.
Furthermore, these findings in this study, which have shed light on the mechanism of ASO-induced neurotoxicity, are likely to deepen understanding of the relationship between toxicity and molecular structure (toxicity-structure correlation) and contribute to the promotion of further technological development of ASOs.
Future Developments
This technology is expected to be fundamental to be applied not only to ASOs but also to other types of nucleic acid medicines, such as small interfering RNA (siRNA), and additional verification is planned to expand its scope of application. Going forward, the research team will utilize these unique technologies to actively work on developing therapeutic drugs for specific diseases.
Notes
The article, “Unraveling and controlling late-onset neurotoxicity of CNS-targeted antisense oligonucleotides through strategic chemical modifications,” was published in Molecular Therapy - Nucleic Acids at DOI: https://doi.org/10.1016/j.omtn.2025.102692.
References
[1] Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, Low A, Bell TA 3rd, Rahdar M, Mukhopadhyay S, Hart CE, Bell M, Riney S, Murray SF, Greenlee S, Crooke RM, Liang XH, Seth PP, Crooke ST. Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat Biotechnol. 2019 Jun;37(6):640-650.
