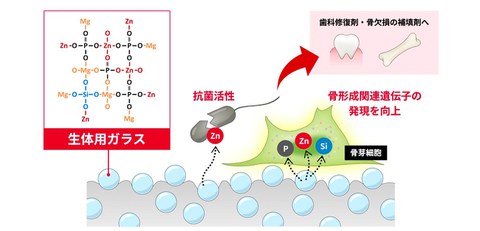
The functionality of bone defect fillers and dental restorative materials are improved
Development of a glass for biomaterials that combines antibacterial properties with bone formation properties
- Development of MgO-ZnO-P₂O₅-SiO₂-based glass composed solely of the orthotetrahedral structures.
- Moderate elution of zinc ions enhances both antibacterial activity and bone formation properties.
- Expected to be a biocompatible material that improves the expression of bone formation-related genes.
*The figure used above is quoted and modified from the original research paper
Credit: Takayoshi Nakano
Outlines
Senior Researcher Sungho Lee at the Multi-Material Research Institute of the National Institute of Advanced Industrial Science and Technology (AIST), in collaboration with Professor Makoto Sakurai of the Department of Applied Chemistry, Graduate School of Engineering at Chubu University, Takayoshi Nakano of the Graduate School of Engineering, the University of Osaka, and Emeritus Professor Toshihiro Kasuga of the Nagoya Institute of Technology, has developed a MgO-ZnO-P₂O₅-SiO₂-based glass that combines antibacterial and bone formation properties as a biomaterial (hereinafter referred to as "bioglass").
When placed in the body, bioglass exhibits bioactivity, directly bonding with bone and biological tissue, and is practically used as a filling material for bone defects, a material for treating periodontal disease, and a material for treating hypersensitive teeth. Among bioglasses, phosphate glass, which is composed primarily of phosphate, can incorporate a variety of elements, which can then be eluted as inorganic ions to activate cell functions. On the other hand, an excessive supply of inorganic ions can inhibit cell function. Among inorganic ions, zinc is known as an essential trace element involved in bone formation and as an element that exhibits antibacterial properties. However, they can be toxic to cells, and their elution behavior must be strictly controlled when developing biomaterials that utilize zinc ions.
In this study, the research group has developed an MgO-ZnO-P₂O₅-SiO₂-based glass that combines excellent antibacterial and bone formation properties by using zinc as a component that forms the glass network structure and controlling the amount of zinc elution. Conventional phosphate glass has a long-chain structure in which phosphate PO₄ tetrahedra are connected via covalent oxygen bonds, but the newly developed glass has a unique glass network structure that does not include long-chain structures formed by covalent oxygen bonds. The results of this research show that the elution of zinc and other components from glass can increase the expression of genes related to bone formation, which is expected to contribute to the development of new biocompatible materials.
Research Background
Biomaterials come into direct contact with living organisms and are used for the purposes of treatment, repair, and functional support. In the 1960s and 1970s, biocompatible materials that do not cause adverse effects when implanted in the body were developed, and since the 1980s, bioactive and bioabsorbable materials have been the focus of research. In recent years, there has been a surge in the development of materials that have tissue regeneration functions through cell activation. Among these, bioglass has been used as a filling material for bone defects and dental restorative materials because it elutes inorganic ions that activate cells and promote bone formation when it comes into direct contact with the living body.
Among bio-glasses, phosphate glass, which is mainly composed of phosphate, can incorporate a wider variety of elements than silicate glass, which is commonly used for window glass and other purposes. Inorganic components incorporated into glass are easily eluted as inorganic ions and exhibit enhanced cell activation properties. However, excessive amounts of inorganic ions can inhibit cell function, so in order to create bioglass using inorganic components that activate cell function and phosphate glass as a base material, it is necessary to strictly control the amount of inorganic ions eluted from the glass.
Research History
AIST is developing bioglass made from phosphate glass as a base material, aiming to create bioglass that has cell activation functions due to inorganic ions. In particular, the researchers are working to develop biocompatible glass that optimizes the ion elution behavior from the material by appropriately designing and controlling the glass network structure, considering the elements to be introduced and their roles in the glass.
Research Contents
The basic units that form the skeletal structure of glass (glass network structure) are phosphate PO₄ tetrahedra and silicate SiO₄ tetrahedra, which have phosphorus (P) or silicon (Si) at the center and oxygen (O) at the four vertices, and these are called network formers (NWFs). On the other hand, alkali metals and alkaline earth metals such as sodium (Na) and calcium (Ca) are called network modifiers (NWMs) and are bonded to the non-bridging oxygen of phosphates and silicates.
Zinc (Zn) and magnesium (Mg) can act as both NWFs and NWMs, but when introduced into glass uncontrolled, they form sixfold coordination of ZnO₆ and MgO₆. This cordination has a weaker bonding strength than phosphorus or silicon, so it does not become a NWF that forms the skeletal structure of glass, but acts as an NWM.
In the newly developed MgO-ZnO-P₂O₅-SiO₂-based glass (SPG-CZ/-MCZ/-MZ), zinc and magnesium form ZnO₄ tetrahedra and MgO₄ tetrahedra, which serve to connect PO₄ tetrahedra and SiO₄ tetrahedra.
Generally, if the NWFs such as phosphates and silicates are not connected to each other, they strongly tend to crystallize, and glass cannot be made. It is difficult to produce glass in which phosphate and silicate coexist in the glass network structure, and glass can only be obtained when one of the components is present in extremely large amounts. Furthermore, phosphate glass can be vitrified even with a composition containing fewer NWFs (lower P₂O₅ content) than silicate glass. The developed glass has a structure in which PO₄ tetrahedra and SiO₄ tetrahedra exist independently without being connected to each other, with ZnO₄ tetrahedra and MgO₄ tetrahedra connecting them, resulting in a structure in which both phosphate and silicate coexist in large numbers within the glass network structure.
The fact that the PO₄ and SiO₄ tetrahedra in the developed glass are orthotetrahedral structures, existing independently and without interconnections, was confirmed by ³¹P and ²⁹Si solid-state nuclear magnetic resonance analysis (solid-state NMR) (Fig. 1). For example, if there is a structure in which two PO₄ tetrahedra are connected, the ³¹P MAS-NMR peak will appear around -10 ppm, and if several PO₄ tetrahedra are connected to form a chain structure, the peak will appear around -20 ppm. Similarly, in ²⁹Si MAS-NMR, a peak appears around -80 ppm when two SiO₄ tetrahedra are connected, and a peak appears around -85 ppm when a chain structure is formed.
In this study, in order to have zinc and magnesium play the role of NWFs, the content of P₂O₅, which forms the glass framework, was drastically reduced, and the composition was controlled so that PO₄ tetrahedra were less likely to connect to each other. As a result, in the SPG-MZ and SPG-MCZ glasses in which zinc and magnesium were simultaneously introduced, the glass network structure was particularly formed solely from orthotetrahedral structures of PO₄, SiO₄, MgO₄, and ZnO₄.
Fig. 1 ³¹P (left) and ²⁹Si (right) solid-state NMR spectra of the developed glass. (SPG-MC: MgO-CaO-P₂O₅-SiO₂ glass (conventional silicophosphate glass), SPG-CZ: CaO-ZnO-P₂O₅-SiO₂ glass, SPG-MCZ: MgO-CaO-ZnO-P₂O₅-SiO₂ glass, SPG-MZ: MgO-ZnO-P₂O₅-SiO₂ glass) Credit: Takayoshi Nakano
While zinc ions induced into glass have the advantage of providing excellent antibacterial and chemical durability, excessive elution of zinc ions into living organisms can be toxic to cells. Therefore, when developing biomaterials using zinc, it is necessary to control the amount of zinc ions eluted within a range that does not cause toxicity to cells. Since the zinc incorporated into the newly developed glass acts as a glass NWF, strongly linking phosphates and silicates in the form of ZnO₄ tetrahedra, it significantly reduces the elution of various ions in the glass, including zinc, into cells. Conventional zinc-free silicophosphate glass (SPG-MC, Fig. 2 a) loses approximately 40% of its ions within seven days. In contrast, the newly developed glass containing an appropriate amount of zinc (Fig. 2 b-d, SPG-CZ/-MCZ/-MZ) showed a dissolution rate of 3% or less for various ions in the glass after immersion for up to seven days, which was less than one-tenth of that of conventional glass. The amount of zinc ions eluted from the developed glass (SPG-CZ/-MCZ/-MZ) was 0.08 mM or less, which was lower than the 0.1 mM concentration required for 50% inhibition of cell proliferation. Furthermore, a proliferation assessment of human osteoblasts confirmed that the eluted zinc ions did not cause cytotoxicity.
Fig. 2 Ion elution rate after immersing the developed glass in Tris buffer solution (pH 7.4) for a specified period of time. (SPG-MC: MgO-CaO-P₂O₅-SiO₂ glass (conventional silicophosphate glass), SPG-CZ: CaO-ZnO-P₂O₅-SiO₂ glass, SPG-MCZ: MgO-CaO-ZnO-P₂O₅-SiO₂ glass, SPG-MZ: MgO-ZnO-P₂O₅-SiO₂ glass) Credit: Takayoshi Nakano
In order to confirm the antibacterial properties of zinc ions, the research group examined the number of bacteria after culturing Escherichia coli and Staphylococcus aureus for 24 hours in the presence of zinc-containing glass (Fig. 3). If the number of bacteria is reduced by more than two digits of magnitude compared to the number of bacteria cultured without glass (Control in the figure), it can be determined that the product has antibacterial properties. The zinc-free glass (SPG-MC) showed the same bacterial count as Control, indicating that it has no antibacterial properties. On the other hand, the newly developed zinc-containing glass (SPG-CZ/-MCZ/-MZ) not only reduced the number of bacteria by more than two orders of magnitude on the graph but also showed a value close to the lower limit that could be counted by the device (Blank: measured using only culture medium without bacteria). In order to obtain a more precise count of bacteria, the number of colonies formed on an agar medium was counted (see tables in Fig. 3). The results showed that the bacteria count was reduced by approximately six orders of magnitude, demonstrating excellent antibacterial properties.
Fig. 3 The developed glass (SPG-CZ/-MCZ/-MZ) and the number of (a) Escherichia coli and (b) Staphylococcus aureus after 24 hours of cultivation. (Blank: no bacteria, Control: with bacteria, Sample name: bacteria + each glass sample) Credit: Takayoshi Nakano
The research group also investigated its bone formation promoting function. At each stage of bone formation by osteoblasts, the genes that are typically expressed are type I collagen (Col I) in the early stage, alkaline phosphatase (ALP) and osteopontin (OPN) in the middle stage, and osteocalcin (OCN) in the late stage. By measuring the expression levels of these genes, the effect of promoting bone formation can be evaluated. Generally, if the value is 1.5 times or higher than the targets for comparison, it can be determined that gene expression has been promoted. Human osteoblasts cultured in a medium containing ions eluted from the developed glass (SPG-CZ/-MCZ/-MZ) showed gene expression levels of all bone formation-related genes that were more than 50 times higher for ALP in the middle stage and OCN in the late stage compared to the Control (using an osteogenic differentiation-inducing medium that accelerates bone formation) (Fig. 4). Furthermore, the zinc-containing glass showed significantly higher gene expression levels than the zinc-free glass. In particular, OCN, which is expressed in the late stage of bone formation, showed gene expression levels approximately 1.8 times higher in zinc-containing glass than in zinc-free glass. These results demonstrate that the developed glass has both antibacterial and bone formation-promoting properties, and that a glass network structure has been designed that allows the incorporated zinc to be eluted at an appropriate concentration.
Fig. 4 Relative expression levels of bone formation-related genes after culturing human osteoblasts for two weeks using cell culture medium containing ions extracted from the developed glass (SPG-CZ/-MCZ/-MZ).
(a) Type I collagen (Col I), (b) alkaline phosphatase (ALP), (c) osteopontin (OPN), (d) osteocalcin (OCN).
(Control: Osteogenic differentiation induction medium, Sample name: Medium containing inorganic ions eluted from each glass)
Future Development
In the future, the researchers will utilize the antibacterial properties and bone formation-promoting effects of the developed glass to create dental materials and cell scaffold materials for tissue engineering.
Notes
The article, “Preparation of Bifunctional Orthosilicophosphate MgO-CaO-ZnO-P₂O₅-SiO₂ Glasses: in vitro Evaluation of Antibacterial Activity and Osteoblast Gene Expression Behavior,” was published in Advanced Healthcare Materials at DOI: 10.1002/adhm.202502546.
