\The “buds” are open and the transport begins/ Discovery of a cap structure at the tip of the flagellar export channel complex
Potential as an antibacterial drug target
- The researchers have elucidated a high-resolution three-dimensional structure of flagellar protein-export channel complex by using cryoelectron microscopy (cryoEM).
- The FliPQR complex, formed by FliP, FliQ, and FliR, which constitute the flagellar protein export, has both an entrance gate and an exit gate, and its opening and closing are strictly controlled. However, the opening and closing mechanisms and the initiation of flagellar formation have long remained unknown.
- The research team discovered a cap structure resembling the edge of a closed bud at the tip of the channel's exit gate, and elucidated the exquisite mechanism by which the channel's exit gate opens in stages, just like the petals of a flower opening one by one, to transport flagellar proteins and initiate flagellar formation.
- Progress in understanding protein-export channel involved in bacterial motility and pathogenicity are expected to develop new antibacterial agents and application in nanotechnology.
Outlines
A collaborative research group including Associate Professor Tohru Minamino of the Protonic NanoMachine Group in the Graduate School of Frontier Biosciences, the University of Osaka, Specially Appointed Assistant Professor Miki Kinoshita (Full Time), Specially Appointed Associate Professor Tomoko Miyata (Full Time), Guest Associate Professor Fumiaki Makino (JEOL Ltd.), Specially Appointed Professor Keiichi Namba (Full Time) of the JEOL YOKOGUSHI Research Alliance Laboratories at the same graduate school, and Professor Katsumi Imada of the Graduate School of Science at the same university, has elucidated the structure of the exit gate of the export channel complex necessary for producing the bacterial locomotory organelle, the flagellum, and revealed, for the first time in the world, the mechanism by which the gate opens like a flower blooming, initiating flagellar formation.
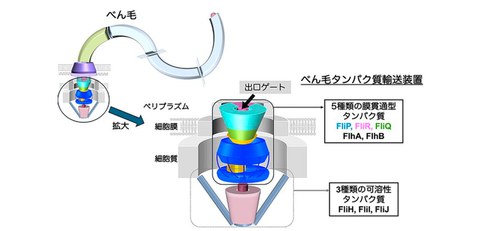
Many motile bacteria have a protein-based motility organelle called a flagellum on their cell surface, which allows the bacteria to move through various environments. Flagella are formed by the stepwise assembly of approximately 30 types of proteins. A specialized protein export located at the base of the flagellum sequentially sends the flagellar components formed inside the cell out of the cell. This export consists of five different transmembrane proteins (FlhA, FlhB, FliP, FliQ, and FliR) and three soluble proteins (FliH, FliI, and FliJ). Among them, FliP, FliQ, and FliR form the FliPQR complex that acts as a export channel complex that transports flagellar proteins out of the cell membrane (Fig. 1). The FliPQR complex has an entrance gate on the cytoplasmic side and an exit gate on the periplasmic side, respectively, and the opening and closing of these gates is strictly controlled. However, its open/close mechanism and how the first flagellar component, called FliE, is positioned to initiate flagellar formation, remained unknown for a long time.
In this study, by using cryoelectron microscopy (cryoEM), the collaborative research team discovered a β-sheet-like "cap" structure at the tip of the FliPQR export channel complex, which they named the β-cap (Fig. 2). The researchers have revealed that the β-cap not only plays a role in keeping the exit gate of the export channel tightly closed but also functions to guide the first transported FliE subunit to the starting point of flagellar formation. Furthermore, the researchers also elucidated the mechanism by which successively transported FliE subunits induce structural changes in the β-cap, causing the exit gate to open stepwise, like a flower blooming, and flagella formation begins.
Because flagella are deeply involved in infection by pathogenic bacteria and biofilm formation, these discoveries are expected to lead to a better understanding of pathogenic mechanisms and the discovery of new antibacterial targets.
Fig. 1 Schematic diagram of a bacterial flagellum and the specialized protein-export channels at its base
Credit: Tohru Minamino
Research Background
Previous research has revealed that the flagellar protein-export consists of five FliP and one FliR subunits assembled in the cell membrane to form the FliPR complex, and that four FliQ subunits bind to the surroundings to form the protein-export channel complex. This transport channel complex has gate structures on both the cytoplasmic and periplasmic sides. Of these, the exit gate on the periplasmic side must be completely closed before flagellar formation begins, that is, until the FliE subunit, the first flagellar protein to be transported, is exported. However, in the structure of the export channel complex revealed so far by cryoelectron microscopy (cryoEM), the structure near the tip of the exit gate was destructed and open. Therefore, the open/close mechanism of gates to initiate flagellum formation has not clarified.
Research Contents
The joint research group assumed that the cause of the destruction of the exit gate of the export channel complex was the surfactant used during cryoEM, so they reconstituted the export channel complex into a peptidisc and analyzed its structure using cryoEM. As a result, the researchers were able to visualize the entire exit gate, the structure of which had previously been unknown, at high resolution and successfully constructed an atomic model (Fig. 2). At the tip of the exit gate, amino acid sequences of five FliP subunits and one FliR subunit each form a β structure, which assembles to form a lid structure resembling the tip of a flower bud, called the β-cap. The research team clarified that this β-cap keeps the exit gate tightly closed.
Furthermore, they discovered that the β-cap has a small gap near the starting point of flagellar formation that allows only one FliE subunit to pass through, which is the initial transport molecule during flagellar formation. This also revealed that the β-cap functions to guide the first FliE subunit to the starting point of flagellar formation. They also found that when the transported FliE is fixed, the β-cap and part of the export channel complex open, resulting in the formation of a new binding site for the next FliE subunit to be incorporated. From these above-mentioned results, they have discovered a new opening mechanism in which the exit gate opens in stages, synchronized with FliE transport, like the petals of a flower bud opening one by one (Fig. 3).
This study is the first in the world to elucidate the mechanism by which the exit gate of an export channel opens, something that had not been clarified for many years.
Fig. 2 Three-dimensional structure of the FliPQR complex reconstituted into a peptidisc
Credit: Tohru Minamino
Fig. 3. Mechanism of exit gate opening of the FliPQR export channel complex upon FliE binding
Credit: Tohru Minamino
Social Impact of the Research
Salmonella infections affect 75 million people worldwide each year, killing approximately 27,000 people annually. Antibacterial agents are widely used to treat bacterial infections, but in recent years, the emergence of multidrug-resistant bacteria has led to an increasing number of cases in which conventional treatments are ineffective. According to a report by the World Health Organization (WHO), the number of deaths from bacterial infections is expected to exceed that of cancer by 2050, making the development of novel treatment strategies to replace existing antibacterial agents an urgent challenge.
Bacterial flagellar motility is known to be deeply involved in the expression of pathogenicity and biofilm formation. The flagellar protein export is a molecular mechanism unique to bacteria and is not essential for bacterial survival. Therefore, if a drug that can selectively inhibit only this function is developed, it may be possible to effectively suppress the flagellar motility and infectivity of pathogenic bacteria.
The results of this research are expected to lead to further progress in research into the unsolved issue of the open/close mechanism of the entrance gate. The detailed three-dimensional structure of the FliPQR export channel complex has also been elucidated, so drug screening directly targets this mechanism is now become possible. In the future, it is expected that identifying compounds that bind to and inhibit the function of the FliPQR complex will lead to significant advances in the development of new therapeutic drugs that can help control novel bacterial infections.
Notes
The article, “A β-cap on the FliPQR protein-export channel acts as the cap for initial flagellar rod assembly,” was published in American scientific journal of Proceedings of the National Academy of Sciences of the United States of America (online) at DOI: https://doi.org/10.1073/pnas.2507221122
