Elucidation of the mTORC1 pathway regulatory mechanism which senses internal nutritional status
Selective dephosphorylation of TSC2 on lysosomal membranes accurately regulate mTORC1 activity
- Protein absolute quantification (iMPAQT) of mTORC1 pathway (Fig. 1) and comprehensive identification of proteins on the lysosomal surface membrane (Lyso-BioID) were conducted by using mass spectrometry (LC-MS/MS) analysis.
- Based on the measurement data, a mathematical model of the mTORC1 pathway was established and elucidated the mTORC1 pathway regulatory mechanism is activated.
- While AKT stimulated by insulin phosphorylates TSC2, inhibiting the braking function (GAP activity) of TSC1/2, the research group found that the phosphorylation level of TSC2 was significantly lower when amino acids were removed than when amino acids were added (Fig. 2 and 5).
- The research group found that when amino acids were translocated, TSC2 moves to the lysosomal membrane and is selectively dephosphorylated by PP2A (Fig. 3, 4 and 5D).
- The regulation of the mTORC1 pathway via PP2A-TSC2 is a system that ensures mTORC1 is activated only when it simultaneously senses amino acids and a growth factor (insulin), and it was shown that its disruption can lead to the development of diseases such as cancer and tuberous sclerosis (Fig 4.and 5).
Outlines
A research group including Assistant Professor Takanori Nakamura, Professor Junya Masumoto, and Professor Tatsuya Sawasaki of Premier Institute for Advanced Studies (PIAS) Proteo-Science Center (PROS) at Ehime University, in collaboration with Professor Mutsuhiro Takekawa of the Institute of Medical Science, The University of Tokyo, Specially Appointed Professor Masato Okada of the Center for Advanced Modalities and DDS, the University of Osaka, Specially Appointed Professor Takashi Suzuki (full-time) of Center for Mathematical Modeling and Data Science, the University Osaka, Professor Masaki Matsumoto of the Niigata University Graduate School of Medical and Dental Sciences, and Professor Naohiko Koshikawa of the School of Life Science and Technology, Science Tokyo (also affiliated with Kanagawa Cancer Center), has succeeded in elucidating the regulatory mechanism of the protein complex mTORC1, which plays a central role in nutrient signaling.
mTORC1 is activated on the lysosomal membrane by the amino acid pathway and insulin pathway (AKT-TSC1/2-Rheb), and determines whether to synthesize or degrade biological components such as proteins, lipids, and nucleic acids depending on the nutritional status of the body, thereby maintaining homeostasis. Although activation of mTORC1 is strictly regulated since disruption of mTORC1 causes metabolic diseases such as cancer and diabetes, the detailed molecular mechanism behind this remains largely unknown. In this study, the research group aimed to achieve a comprehensive understanding of the mTORC1 signaling pathway by utilizing experimental techniques such as a proximity-labeling method (Lyso-BioID) and theoretical methods (mathematical analysis). As a result, AKT kinase after insulin stimulation was activated regardless of intracellular amino acid concentration, but the researchers discovered that at low amino acid levels, TSC2, a substrate molecule for AKT that acts as a brake on the mTORC1 pathway, is selectively dephosphorylated by the phosphatase PP2A on the lysosomal membrane. This dephosphorylation reaction was revealed to be one of the molecular mechanisms that ensures mTORC1 is activated only when it simultaneously senses amino acids and insulin. In addition, they have found genetic mutations that eliminate the PP2A-binding ability of TSC2 in cases of tuberous sclerosis (designated intractable disease 158) and cancer, and have also elucidated the disruption of the braking function of the mTORC1 pathway by PP2A-TSC2 leads to the development of the above-mentioned TSC2-related diseases.
Research Background
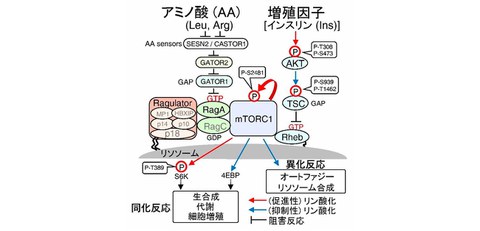
The cells that make up the human body maintain their biological activities by appropriately regulating the synthesis (anabolism) of macromolecules such as proteins, nucleic acids, and lipids that create the body, and the breakdown (catabolism) of these macromolecules during starvation, using stimulation from growth factors (e.g., insulin), extracellular nutrient sources such as amino acids, or intracellular energy. mTORC1 (mTOR complex 1) is known to be a key molecule that determines whether a cell will induce anabolic or catabolic reactions depending on the nutritional status. mTORC1 is a protein complex centered around the kinase mTOR, which is highly conserved across species. It promotes anabolic reactions by phosphorylating regulatory proteins involved in the biosynthesis of proteins, lipids, nucleic acids, etc. On the other hand, by phosphorylating molecules involved in autophagy and lysosomal synthesis, it inhibits the catabolic reactions that degrade biopolymers to produce nutrients (Fig. 1).
Fig. 1 mTORC1 nutrient signaling pathway
mTORC1 is activated by active RagGTP and active RhebGTP distributed on the lysosomal membrane surface.
Rag and Rheb are inactive states in the absence of nutrient signals. Therefore, for mTORC1 to be activated, Rag and Rheb must first be activated by the amino acid-sensing pathway (Ragulator-Rag) and growth factor (insulin)-sensing pathway (AKT-TSC1/2-Rheb). RhebGTP is maintained in an inactive state RhebGDP by TSC1/2, which has GAP activity. However, when TSC2 is phosphorylated by AKT activated kinase after insulin stimulation, the GAP activity of TSC2 is suppressed, thereby activating Rheb on the lysosomal membrane. When cells sense nutrients (amino acids), active RagGTP is assembled on the lysosomal membrane via the Ragulator (p18-p14-MP1-p10-HBXIP complex). It has also been reported that Ragulator-RagGTP promotes the translocation of mTORC1 to lysosomes, resulting in the activation of mTORC1 on the lysosomal membrane.
In this way, mTORC1 appropriately senses intracellular amino acid or insulin concentrations to control the on/off of anabolic/catabolic reactions. Therefore, the mTORC1 pathway regulatory mechanism which senses internal nutritional status is known to cause the onset of diseases such as cancer and diabetes. For example, the insulin-sensing (AKT-TSC1/2-Rheb-mTORC1) pathway includes oncogenes (AKT, mTOR) that frequently mutate in cancer, as well as tumor suppressor genes (PTEN, TSC1/2), making it an important signaling pathway involved in the development and progression of cancer and a potential drug target. In recent years, extensive research has been conducted around the world into the mTORC1 pathway regulatory mechanism, and the full picture is beginning to emerge. However, many aspects of the detailed molecular control mechanism remain unknown, such as whether it properly controls multiple signal transduction pathways, such as amino acid and insulin pathways, without crosstalk.
Credit: Takashi Suzuki
Research Contents
The research group aimed to gain a comprehensive understanding of the mTORC1 nutrient signaling pathway by utilizing experimental techniques such as protein absolute quantification (iMPAQT) and identifying lysosomal membrane surface proteins (Lyso-BioID), as well as theoretical methods (mathematical analysis). As a result, AKT kinase in the insulin-sensing (AKT-TSC1/2-Rheb) pathway was activated by insulin stimulation regardless of intracellular amino acid concentrations (Fig. 2A).
On the other hand, the researchers found that the inhibitory phosphorylation of TSC2 by AKT was significantly lower under amino acid removal conditions than under amino acid addition conditions (Fig. 2A). Furthermore, they discovered the reason for the low TSC2 phosphorylation level is that upon amino acid removal state, TSC2 translocates to the lysosomal membrane and is selectively dephosphorylated by the phosphatase PP2A (Fig. 2B, Fig. 3, Fig. 4A, B, Fig. 5). This system of dephosphorylation of TSC2 by PP2A on the lysosomal membrane has been revealed to be one of the molecular control mechanisms that ensures mTORC1 is activated only when cells simultaneously sense amino acids and insulin. Furthermore, the researchers discovered that in tuberous sclerosis (designated intractable disease 158) and cancer cases, genetic mutations such as L366P (tuberous sclerosis), E366K (colorectal cancer, ovarian cancer), and E1583K (skin cancer) occurred in the PP2A-B56 binding domain ([L/M/F/I]-x-x-[I/L/V]-x-E-x) of the TSC2 molecule (Fig. 4C, D). They have also revealed from these results that disruption of the braking function of the mTORC1 pathway by PP2A-TSC2 leads to the development of the above-mentioned TSC2-related diseases.
Fig. 2 TSC2 phosphorylation after insulin stimulation decreases upon removal of amino acid
(A) Cells were deprived of amino acids for one hour, and then stimulated with amino acids and insulin [AA(+) Ins(+)], or with amino acids [AA(+) Ins(–)] or insulin [AA(–) Ins(+)] alone. The time course of changes in the phosphorylation levels of AKT, TSC2, S6K, and S6 was monitored.
(B) The decrease in TSC2 phosphorylation (lane 5) during AA(–) Ins(+) was restored to the TSC2 phosphorylation level observed during AA(+) Ins(+) by the addition of the PP2A inhibitor okadaic acid (OA) (lanes 6 and 7).
Credit: Takashi Suzuki
Fig. 3 Lysosomal localization of TSC2 increases upon amino acid deprivation [AA(–)], whereas PP2A consistently shows lysosomal localization regardless of amino acid or insulin concentration
(A) Microscopic images of the intracellular localization of TSC2, PP2A-Aa/b, and LAMP1 (lysosomal marker) under AA(–) Ins(–), AA(+) Ins(+), AA(+) Ins(–), and AA(–) Ins(+) conditions were taken.
(B) TSC2 showed lysosomal localization upon amino acid deprivation [AA(–)].
(C) PP2A consistently showed lysosomal localization regardless of amino acid or insulin concentration.
Credit: Takashi Suzuki
Fig. 4 PP2A and various phosphatases are distributed on the lysosomal membrane
(A) Comprehensive biotin labeling of proteins on the lysosomal membrane using the proximity-labeling method (Lyso-BioID)
(B) Mass spectrometry (Lyso-BioID) results of proteins distributed on the lysosomal membrane in the presence of amino acids [AA(+)] and in the absence of amino acids [AA(–)].
(C) There are eight PP2A-B56 binding domains ([L/M/F/I]-x-x-[I/L/V]-x-E-x) within the TSC2 molecule.
(D) The PP2A-B56 binding domain ([L/M/F/I]-x-x-[I/L/V]-x-E-x) was found to have the L361P mutation in tuberous sclerosis, the E366K mutation in colorectal cancer and ovarian cancer, and the E1583K mutation in skin cancer.
Credit: Takashi Suzuki
Fig. 5 PP2A-mediated dephosphorylation of TSC2 on the lysosomal membrane regulates mTORC1 activity
A) mTORC1 is inactive in the absence of amino acids (AA) or insulin (Ins) [AA(–) Ins(–)].
(B) When the amino acid (AA)-sensing pathway (Ragulator-Rag) and the insulin (Ins)-sensing pathway (AKT-TSC1/2-Rheb) are simultaneously activated [AA(+) Ins(+)], mTORC1 is translocated to the lysosomal membrane by Ragulator-RagGTP and activated by RhebGTP.
(C) When only the amino acid pathway is activated [AA(+)Ins(–)], Ragulator-RagGTP induces mTORC1 translocation to the lysosome, but mTORC1 is no activated because Rheb is inactive.
(D) When only the insulin pathway is activated [AA(–) Ins(+)], TSC2 is inhibitory phosphorylated by AKT in the cytoplasm, but is translocated to the lysosomal membrane and dephosphorylated by PP2A.
Dephosphorylation restores the GAP activity (brake function) of TSC2, thereby maintaining mTORC1 in an inactive state.
Credit: Takashi Suzuki
Social Impact of the Research
This research clarified that PP2A-TSC2 suppresses mTORC1 activity at low amino acid levels. Furthermore, it also revealed that disruption of PP2A-TSC2 regulation of mTORC1 activity leads to the development of tuberous sclerosis (designated intractable disease 158) or cancer.
In addition, in many cancer cells, AKT and PI3K are constitutively activated due to genetic mutations. In these cancer cells, the insulin-sensing (AKT-TSC1/2-Rheb) pathway remains constantly activated even in the absence of insulin stimulation, and downstream mTORC1 activity is also maintained at a constitutively high level, leading to the progression of cancer. Based on the results of this study, it is thought that new cancer treatments such as amino acid removal therapy may be effective against these cancer cells.
Notes
The article, “Amino acid-dependent TSC2 dephosphorylation by lysosome-PP2A regulates mTORC1 signaling transduction,” was published in Life Science Alliance at DOI: 10.26508/lsa.202503206.
