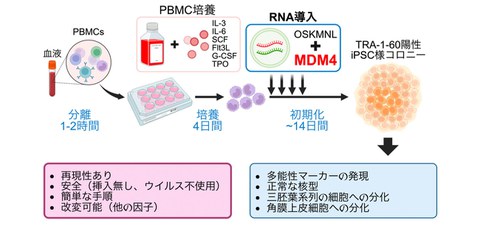
Development of a highly efficient iPS cell generation method from human peripheral blood-derived mononuclear cells (PBMCs)
Modulation of the p53 pathway improves reprogramming efficiency by more than tenfold
・Successful reprogramming of PBMCs with RNA
For the first time, the research group has succeeded in generating iPS cells using synthetic RNA from peripheral blood-derived mononuclear cells (PBMCs), which had previously been considered difficult.
・Suppression of the p53 pathway has dramatically improved reprogramming efficiency
The research team demonstrated that the introduction of MDM4, which suppresses the function of p53, significantly improved the efficiency of RNA reprogramming in PBMCs. In particular, MDM4 added with a mutation that makes it less susceptible to ubiquitin-dependent proteolysis showed the greatest effect.
・The PBMC-derived iPS cells can be differentiated into corneal cells
PBMC-derived iPS cells reprogrammed with synthetic RNA have the potential to differentiate into all three germ layers. In particular, the research team was successful in inducing differentiation into corneal epithelial cells, demonstrating their pluripotency.
Outlines
A research group, including Junior Associate Professor Masato Nakagawa (Center for iPS Cell Research and Application (CiRA) at Kyoto University concurrently serving as Specially Appointed Associate Professor (Full-time) at Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe) at the University of Osaka) has established a method for highly efficient generation of iPS cells (induced pluripotent stem cells) from human peripheral blood-derived mononuclear cells (PBMCs) using synthetic RNA. This study presents a solution to the previously difficult task of non-viral iPS cell generation from human blood cells, and is expected to be applied to future regenerative medicine and personalized medicine.
The results of this study were published online in Springer Nature's open access scientific journal, Scientific Reports, on September 8, 2025 (UK time). After the publication, a protocol (in both Japanese and English) for reprogramming somatic cells (human fibroblasts (HDFs) and PBMCs) using the synthetic RNA developed in this research is planned to be posted on the CiRA website.
Schematic diagram
Credit: Masato Nakagawa
Research Background
iPS cells can be generated by reprogramming various somatic cells and are widely used in regenerative medicine, drug discovery, and disease modeling. Peripheral blood-derived mononuclear cells (PBMCs) are minimally invasive and easy to collect, making them a particularly promising source of iPS cells for establishing from patients. However, PBMCs are vulnerable to the stress associated with external gene introduction, resulting in the death of many cells. Therefore, reprogramming using RNA transfer (RNA reprogramming), which has been attracting attention as a safe, non-viral, non-genetically modified method, has been considered technically difficult. However, RNA reprogramming does not leave any mutations in the genome, and is expected to be standardized in the future as a highly safe technology toward clinical application. In response to this, the purpose of this study was to identify the barriers to RNA reprogramming in PBMCs and develop methods to overcome them. In particular, the researchers focused on the overactivation of the stress response p53 pathway and examined whether its regulation could improve the efficiency of RNA reprogramming.
Research Contents
In this study, the research group developed a new method for generating iPS cells from human peripheral blood-derived mononuclear cells (PBMCs) using synthetic RNA. Previously, PBMCs had low reprogramming efficiency, making it difficult to generate iPS cells using synthetic RNA. Following results are obtained in this research:
1) Introduction of MDM4, previously recognized as a negative regulator of p53, dramatically improved reprogramming efficiency
In particular, MDM4 with the S367A mutation (MDM4-S367A), which prevents ubiquitin-dependent proteolysis, showed the greatest effect, increasing reprogramming efficiency by more than tenfold.
In addition to the reprogramming factor (OSKMNL), synthetic mRNAs for mCherry, p53-R175H, MDM2, or MDM4 were introduced into PBMCs for reprogramming, and the emerged iPS cell-like colonies were stained with the pluripotency marker (undifferentiation marker) TRA-1-60 antibody, and the number of colonies was counted (left graph). Based on this value, the doubling amount was calculated, assuming that the amount obtained when mCherry was added was one (right graph). When MDM4 was added, a doubling of the amount by more than tenfold was observed.
Credit: Masato Nakagawa
2) Reproducibility confirmed with PBMCs derived from multiple donors
The efficacy of MDM4-S367A was consistently observed when reprogrammed with PBMCs from eight healthy donors.
In addition to the reprogramming factor (OSKMNL), synthetic RNAs for d2EGFP, MDM4-WT (wild type), MDM4-S367A (non-phosphorylated form), and MDM4-S367D (phosphorylation mimetic type) were introduced into PBMCs for reprogramming. The emerged iPS cell-like colonies were stained with the TRA-1-60 antibody (left photo, iPS cell-like colonies appear green), and the counted number of colonies (upper right graph). Based on this value, the doubling amount was calculated, assuming that the amount obtained when d2EGFP was added was one (bottom right graph). Immunostaining showed results from three donors. The colony counting experiment used data from eight donors.
Credit: Masato Nakagawa
3) Multifaceted verification of the quality of established iPS cells
The PBMC-derived iPS cells established by RNA reprogramming expressed undifferentiated markers, exhibited the ability to differentiate into three germ layers, and had a normal karyotype, confirming that they possessed the properties of pluripotent stem cells.
Photos of iPS cells established from PBMC using synthetic RNA (left, top two photos) and the karyotyping results (left, bottom two photos). Both were confirmed to have normal karyotypes. These iPS cells and previously established iPS cells (201B7, 1383D2) that have been used in numerous papers were cultured under conditions that maintain undifferentiation (bFGF(+) in the right graph) and under conditions that allow spontaneous differentiation into cells of the three germ layers (bFGF(-) in the tight graph). The results of RT-qPCR were used to verify gene expressions (four graphs on the right). Under bFGF(-) conditions, the expression of three germ layer markers was confirmed.
Credit: Masato Nakagawa
4) Differentiation into corneal epithelial cells was induced, demonstrating the possibility of clinical application
The established PBMC-derived iPS cells were induced to differentiate into corneal epithelial cells, and the expression of markers such as KRT12 and PAX6 was confirmed, suggesting their potential application in visual regenerative medicine.
Corneal epithelial tissue was generated using iPS cells established from PBMCs using synthetic RNA.
Undifferentiated iPS cells (top left photo, MN328) and a differentiated corneal two-dimensional tissue assembly (SEAM) (top, second photo from the left, MN328). It was confirmed that PAX6-positive and TP63-positive corneal epithelial progenitor cells were induced in the third region (3rd) of the SEAM (four photos on the bottom left, immunostaining data). The progenitor cells were isolated and KRT12- and PAX6-positive corneal epithelial tissue was generated (four photographs on the right, immunostaining data).
Credit: Masato Nakagawa
Consideration
It was suggested that the efficiency of RNA reprogramming in PBMCs may be largely limited by the induction of apoptosis due to excessive activation of the p53 pathway.
By using the MDM4 mutant (S367A) in combination, the researchers were able to overcome the reprogramming barrier specific to PBMCs and significantly improved the reprogramming efficiency.
This method is highly reproducible and is practical technology (confirmed in other labs. and facilities).
Since the clinical suitability of the RNA reprogramming method and the improved safety when used in combination with MDM4 have been confirmed, it is thought that this method will also be suitable for future applications in regenerative medicine.
Future Development
In the future, the research group plans to develop disease models using iPS cells obtained from PBMCs and proceed to apply them to personalized medicine. It is also hoped that this protocol will be used in clinical settings as a safer and more practical method for producing iPS cells.
Notes
The article, “MDM4 enables efficient human iPS cell generation from PBMCs using synthetic RNAs,” was published in Scientific Reports at DOI: 10.1038/s41598-025-16446-y.
