Exploratory research of predictive biomarkers for WT1-targeted chemoimmunotherapy for unresectable advanced pancreatic cancer based on long-term survival cases
- WT1-targeted chemoimmunotherapy was administered to ten patients with advanced pancreatic cancer deemed inoperable, and in seven cases, the disease either shrank or was stable for a long period of time, making surgery possible.
- Of these, four patients were able to survive for more than five years, and three patients have survived for more than six years without recurrence or metastasis.
- It was found that long-term-surviving patients already had an immune response that reacted to WT1 before treatment, which serves as a biomarker for predicting treatment efficacy.
- In particular, there was an increase in WT1-specific memory CD8+ T cells (central memory CD8+ T cells), which are capable of repeatedly attacking pancreatic cancer cells, suggesting that this may be related to long-term survival.
- It was confirmed that WT1-targeted chemoimmunotherapy induces immune responses not only against WT1 but also against various markers (tumor antigens) present on pancreatic cancer cells.
- The study found that WT1-targeted chemoimmunotherapy may be a promising treatment strategy if patients have an immune response to WT1 before treatment.
Outlines
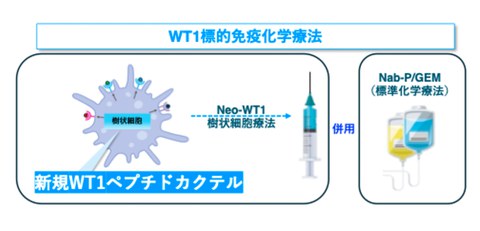
Professor Shigeo Koido of Division of Gastroenterology and Hepatology at the Jikei University School of Medicine (at the time of research) and his research team conducted WT1-targeted chemoimmunotherapy using dendritic cells (Neo-WT1 dendritic cells) using a novel multifunctional peptide (Neo-WT1) in combination with anticancer drugs (nab-paclitaxel plus gemcitabine, Nab-P/Gem) against Wilms’ tumor 1 (WT1) gene which was co-developed with the University of Osaka Graduate School of Medicine, Tokyo Midtown Advanced Medical Research Institute, Kanazawa Medical University, and Juntendo University.
As a result, in seven out of ten cases of advanced pancreatic cancer that were deemed inoperable, the tumors shrank or remained stable for a long period of time, making surgery possible. Of these, four patients were able to survive for more than five years. In this study, the researchers identified several indicators (biomarkers) that can predict treatment efficacy.
Research Background
Advanced pancreatic cancer creates a special environment around the cancer that makes it difficult for the body's immune system to function, making current treatments such as chemotherapy, radiation therapy, and immune checkpoint inhibitors less effective. However, a new treatment called chemoimmunotherapy has been attracting attention recently, which targets the environment surrounding the cancer (also called the tumor microenvironment) itself, and is expected to become a new treatment option for patients with unresectable advanced pancreatic cancer.
WT1 is known to be expressed in large amounts in pancreatic cancer cells, cancer stem cells, blood vessels that supply nutrients to cancer, and cells that suppress immune function. Therefore, WT1 is attracting attention as a therapeutic target for various cancers. The research group has developed Neo-WT1 dendritic cell therapy by using a new type of WT1 peptide (Neo-WT1) that differs from conventional WT1, and by providing Neo-WT1 information to cells that activate the immune system (dendritic cells), which are then administered to patients with pancreatic cancer. Then the research group has been the first in the world to devise and implement WT1-targeted chemoimmunotherapy, which combines Neo-WT1 dendritic cell therapy with currently used chemotherapy (nab-paclitaxel plus gemcitabine, Nab-P/Gem) (see figure below).
This treatment method can stimulate strong immune responses against WT1, a cancer marker, and has enabled patients with unresectable advanced pancreatic cancer to completely remove the cancer (R0 resection). However, because the effectiveness of WT1-targeted chemoimmunotherapy is thought to depend on various factors, such as each individual's immune response, it has been difficult to accurately predict how this treatment will work for each patient.
Credit: OKA Yoshihiro
<WT1-targeted chemoimmunotherapy schedule>
The first course consisted of standard chemotherapy (nab-paclitaxel plus gemcitabine, Nab-P/Gem) alone, and from the second course, Neo-WT1 dendritic cells were used in combination with Nab-P/Gem. The administration of Neo-WT1 dendritic cells was limited to 15 times (see figure below).
Purpose/Procedure
A protein called WT1 was expressed in a lot of pancreatic cancer cells. The treatment that combines immunotherapy, which targets and attacks WT1, with anticancer drugs is called WT1-targeted chemoimmunotherapy. To predict how effective this new treatment would be, the research group took a closer look at what changes were occurring in human body and what the state of the cancer was. In this study, the researchers investigated the following in nine cases of unresectable advanced pancreatic cancer.
- The number of WT1-specific CD8+central memory T cells that can immediately act when encountering cancer again exist among the immune cells (killer CD8+ T cells) that react to WT1 and attack cancer.
- The number of WT1 antibodies exist in the body to attack WT1-expressing cancers.
- The types and numbers of immune cells gather around the cancer.
- The changes occur in circulating tumor DNA (ctDNA) of cancer cells circulating in the blood.
- The characteristics of pancreatic cancer cells themselves.
Based on above information, the purpose of this study is to clarify which types of patients are likely to benefit from WT1-targeted chemoimmunotherapy.
Results
Treatment outcome
The median progression-free survival (PFS) and overall survival (OS) in the nine patients with unresectable advanced pancreatic cancer were 2.23 years and 3.52 years, respectively (see figure below).
Credit: OKA Yoshihiro
Patients who were able to undergo surgery after their pancreatic cancer had shrunk or stabilized (seven cases) were found to have longer survival times than those who were not able to undergo surgery (two cases). Four patients who were able to undergo surgery survived for more than five years. No recurrence or metastasis was observed in three cases even after six years. The long-term survivor had unresectable locally advanced pancreatic cancer, and surgery was possible as a result of the induction of a strong WT1-specific immune response (see figure below).
Credit: OKA Yoshihiro
Biomarkers for predicting treatment efficacy
Compared with patients with slightly poorer treatment outcomes, patients who achieved long-term survival had the following characteristics:
1. The number of WT1-specific CD8+ naive T cells in peripheral blood was decreased before treatment.
2. High levels of IgM antibodies against WT1 were observed even before treatment.
3. No mutated cancer genes (such as KRAS and TP53) were found in the blood before treatment.
4. In patients who had ctDNA mutations in their blood before treatment, the frequency of these mutations decreased after treatment.
5. Patients whose pancreatic cancer cells do not highly express PD-L1 protein have a long-term survival rate. PD-L1 acts as a "brake" on the activity of immune cells, and when there is less of it, the immune system becomes more effective.
6. Investigating the tissues surrounding pancreatic cancer (tumor microenvironment) revealed that patients who had higher treatment efficacy had large concentrations of immune cells called CD103+ cells (brown cells in the image on the left below) and CD20+ cells (brown cells in the image on the right below), but this analysis did not find any correlation with life prognosis.
7. In patients who had particularly higher treatment efficacy, the number of WT1-specific CD8+central memory T cells in the peripheral blood increased after treatment.
Thus, patients who responded well to WT1-targeted chemoimmunotherapy were already primed for an immune response to WT1 before treatment. Examining these characteristics before and during treatment may help predict who will benefit from this treatment.
Credit: OKA Yoshihiro
Future Applications/Challenges
In the future, to accurately confirm the effectiveness of WT1-targeted chemoimmunotherapy, it is desirable to conduct large-scale clinical trials using chemotherapy alone or immunotherapy alone as comparison controls. Through these studies, it will be possible to scientifically verify whether WT1-targeted chemoimmunotherapy is truly more effective than other treatments. It is also extremely important to develop biomarkers to identify individuals who are particularly likely to benefit from this treatment. For example, it is thought that people who (1) already have activated immune cells (T cells) that react to WT1, or (2) already have antibodies against WT1 before starting treatment, may respond well to this treatment. To prove whether these predictions are correct, comparative studies and clinical trials involving a larger number of patients are needed, which is a major challenge for the future.
Summary
The diagram below summarizes the differences in WT1-specific immune responses between super responders who achieved excellent treatment outcomes with WT1-targeted chemoimmunotherapy and non-super-responders who achieved somewhat poorer treatment outcomes.
<Diagram description>
- Many pancreatic cancers have few immune cells, especially T cells, in the tumor microenvironment, or most tumors are cold tumors in which the immune response is suppressed. However, some tumors may be transformed into hot tumors in which the immune response is activated with WT1-targeted chemoimmunotherapy. Patients with low numbers of WT1-specific CD8+ naive T cells and high levels of WT1-specific IgM antibodies before treatment may benefit from WT1-targeted chemoimmunotherapy.
- Super-responders to WT1-targeted chemoimmunotherapy showed significantly greater increases in WT1-specific CD8+central memory T cells after treatment than non-super-responders.
- Patients whose circulating tumor DNA (ctDNA) contained pancreatic cancer cells that had mutations (i.e., neoantigens) in either of two key genes (KRAS and TP53) and expressed high levels of PD-L1 before treatment had a significantly poorer prognosis.
- After pancreatic cancer is destroyed by chemotherapy or Neo-WT1 dendritic cell therapy, various tumor antigens are released. As a result, IFN-γ producing CD8+ T cells that react to neoantigens and oncoantigens were confirmed (blue arrow in the bottom row of the figure below). In other words, the researchers confirmed that WT1-targeted chemoimmunotherapy induces an immune response to abnormal proteins specific to cancer cells called neoantigens (such as mutated TP53) and oncoantigens (such as WT1, TP53, and KRAS). Therefore, it is expected that this treatment will enable the immune system to recognize, attack, and eliminate pancreatic cancer cells that express neoantigens and oncoantigens. In short, by reacting to various neoantigens and oncoantigens, which are cancer markers, it may be possible that a more powerful mechanism of attacking cancer is at work (see figure below).
Credit: OKA Yoshihiro
Blue arrows at the bottom: the induction of IFN-γ producing CD8+ T cells that react to neoantigens and oncoantigens was confirmed
Credit: OKA Yoshihiro
Notes
The article, “Predictors of patients with advanced pancreatic cancer undergoing conversion surgery via chemoimmunotherapy with a multifunctional Wilms' tumor 1 (WT1) peptide cocktail-pulsed dendritic cell vaccine,” was published in Journal for ImmunoTherapy of Cancer (Online) at DOI: https://jitc.bmj.com/content/13/7/e011426.
Illustration (Credit: OKA Yoshihiro)
