Discovery of molecular patterns that determine the boundary between “self” and “nonself” inside the cell
Elucidated the mechanism by which cell autonomous immunity captures pathogen-containing vacuoles of self-derived components
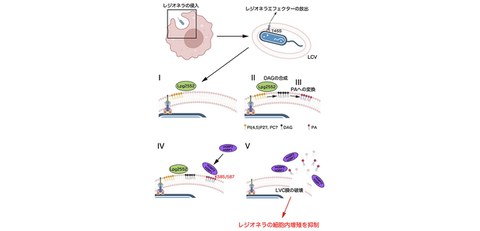
- It is clarified that the Legionella virulence factor Lpg2552 is required for the accumulation of GBP, a cell autonomous immunity-related molecule, in Legionella-containing vacuoles (LCVs), which are covered with host-derived organelle membranes.
- In addition to finding that Lpg2552 promotes the synthesis of phosphatidic acid in LCV membranes, the researchers found that GBP recognizes this accumulation of phosphatidic acid as a sign of nonself and disrupts the LCV membrane. Furthermore, it is found that a GBP mutant that cannot recognize phosphatidic acid cannot capture LCV membranes and, as a result, it is impossible to inhibit the intracellular growth of Legionella.
- These results are expected to support the study of the mechanism by which GBP attacks vacuoles containing other pathogens, and brings a stepping stone to elucidating the mechanism of development of autoimmune diseases involving GBP.
Outlines
A research group of Professor Kohei Arasaki of Tokyo University of Pharmacy and Life Sciences School of Life Sciences, in collaboration with a research group of Professor Masahiro Yamamoto and Associate Professor Miwa Sasai of the Research Institute for Microbial Diseases at the University of Osaka, Professor Hiroki Nagai and Associate Professor Tomoko Kubori of the Gifu University Graduate School of Medicine, and Professor Ichiro Nakagawa and Associate Professor Takashi Nozawa of the Kyoto University Graduate School of Medicine, has elucidated the mechanism by which Legionella-containing vacuole (LCV) membranes, which are covered by the cell membrane, a self-component of the infected host, are captured by the cell autonomous immunity as a nonself organism inside the cell. The results of this research revealed some of the molecular mechanisms by which the cell autonomous immunity distinguishes self-components as "nonself." Analysis of this mechanism is expected to lead to a better understanding of the mechanisms underlying the development of autoimmune diseases that originate from cell autonomous immunity.
The result of this research was published in Proceedings of the National Academy of Sciences of the United States of America by American National Academy of Sciences.
Research Background
In the immune system, it is essential to precisely distinguish “self” from “nonself”. In general, the innate and adaptive immune systems distinguish between self and nonself based on Pathogen Associated Molecular Patterns (PAMPs). On the other hand, in the cell autonomous immunity, which captures and kills pathogens that have invaded cells, the boundary between self and nonself becomes unclear. The reason for this is that many pathogens that invade cells are not exposed but are covered by a host-derived organelle membrane and the cell autonomous immunity captures and attacks pathogens covered by this host-derived organelle membrane. In other words, since the pathogen recognizes and attacks "self-derived" membrane components as "nonself," this discrimination is thought to be based on patterns associated with molecular changes in host factors caused by the pathogen's life cycle, Pathogen "Life-cycle" Associated Molecular Patterns (PLAMPs). Therefore, it is expected that elucidating the type of intracellular molecular changes turning "self" into "nonself" will lead to a better understanding of "PLAMP" in the cell autonomous immunity.
Legionella is a pathogen that has often been reported in the news as infection cases in facilities that handle water, such as hot springs and public baths, and infection can cause serious pneumonia. It may be still in fresh in people’s memories that Legionella exceeding the standard level was detected at the EXPO 2025. Since Legionella bacteria multiply within host cells, they are classified as “intracellular bacteria."
After invading a host cell, Legionella creates Legionella-containing vacuole (LCV) membranes within the cell, which is surrounded by the host-derived organelle membrane. Legionella then controls the dynamics of LCVs, blocking their transport to lysosomes (intracellular degradation factories) and directing them to the endoplasmic reticulum, where they multiply. Legionella releases many virulence factors called "Legionella effectors" into host cells to control the dynamics of LCV.
On the other hand, it is known that human cells have mechanisms that can confront intracellular pathogens. Interferon is a cytokine that promotes the expression of genes that fight against infectious pathogens, and previous studies have clarified that guanylate binding protein (GBP) (GBP2 in mice and GBP1 in humans), a type of GTPase induced by interferon, captures and destroys the LCV membrane. Furthermore, it is shown that LCVs containing mutant strains that are unable to release Legionella effectors are not captured by these GBPs. In other words, it is assumed that these GBPs identify molecular changes on the LCV caused by some Legionella effector, but the details remain unknown.
Research Contents
In this study, the researchers used a system that forced the expression of GBP genes in cultured cells and started to search for Legionella effectors that promote the capture of LCVs by GBP. As a result, they found that GBP capture was not detected in LCVs containing Legionella that lacked the Legionella effector gene, called "Lpg2552." Since Lpg2552 has been reported as a factor that promotes the synthesis of the phospholipid diacylglycerol (DAG) on the LCV, the research group hypothesized and tested the possibility that GBP recognizes DAG on the LCV. However, the results revealed that GBP specifically recognizes another phospholipid, phosphatidic acid (PA), not DAG. Although this result seems to differ from the original hypothesis, it makes sense considering that DAG synthesized in cells is rapidly converted to PA. In fact, analysis using a fluorescent probe that specifically labels PA revealed that PA is accumulated on LCVs in an Lpg2552-dependent manner. These results indicate that GBP recognizes PA accumulated on the LCV by the action of Lpg2552 as a sign of nonself. PAs are usually components of biological membranes such as organelles. Then, the research group analyzed the molecular mechanism by which GBP specifically recognizes only LCV membranes within cells (not organelle membranes), and found that the concentration of PA that can be recognized by GBP is significantly higher than that recognized by normal PA-binding proteins. Therefore, it is thought that GBP considers that the concentration of PA is the boundary between "self" and "nonself". Furthermore, they succeeded in identifying the amino acid residues in GBP that are important for PA recognition (GBP2: lysine residue at position 585, GBP1: lysine residue at position 587). In addition, expression experiments of GBPs with these mutated residues showed that the mutations had a significant effect on LCV capture and the inhibition of intracellular growth of Legionella.
Next, the research group verified whether PA production on LCVs by Lpg2552 is necessary for pathogen removal in the presence of interferon. First, they confirmed that GBP, whose expression level was significantly increased by interferon treatment, captured the LCV membrane and strongly inhibited the intracellular multiplication of Legionella compared to untreated conditions. They also found that LCV containing the Lpg2552 deletion strain escaped from GBP whose expression was induced by interferon and further proliferated significantly even in the presence of interferon.
These results indicate that the elimination of Legionella by cell autonomous immunity utilizes PA accumulated on the LCV by Legionella’s own action.
Research overview
Credit: Masahiro Yamamoto
Future Developments
This study has partially clarified the molecular mechanism that determines the boundary between "self" and "nonself" in the cell autonomous immunity. Considering that the cell autonomous immunity is involved in capturing and eliminating not only Legionella but also many other pathogens that are enveloped by host-derived organelle membranes, the findings of this study will help elucidating the mechanisms by which the cell autonomous immunity captures other pathogens. Furthermore, the findings will be useful in understanding the mechanism of onset of GBP-related autoimmune diseases (e.g., abnormal PA accumulation is detected in these patients).
Notes
The article, “Phosphatidic acid production on the vacuole harboring Legionella pneumophila is a signal for recognition of interferon-induced GTPases,” was published in Proceedings of the National Academy of Sciences of the United States of America.
