Elucidated the mechanism by which laminin and integrin transform macrophages into dendritic cell (DC)-like cells
Application for cancer immunotherapy is expected
- It was found that inhibition of integrin α7 function causes macrophages to acquire dendritic cell (DC)-like morphology and functions.
- The molecular mechanism was elucidated by which integrin α7 inhibition activates the PI3K/AKT pathway and promotes differentiation into dendritic cells.
- It was clarified that cell culture medium using laminin α2 improve the induction efficiency of dendritic cells.
- The researchers indicated that the interaction between laminin α2 and integrin α7 is involved in dendritic cell differentiation, suggesting its potential application in immunotherapy.
Outlines
A research group led by Professor Nagako Yoshiba of the Department of Oral Health and Welfare, Graduate School of Medical and Dental Sciences, Niigata University, and Research Professor Tomoki Maekawa of the Advanced Oral Function Education and Research Center at the same graduate school, in collaboration with Endowed Research Chair Professor Kiyotoshi Sekiguchi of the Institute for Protein Research, University of Osaka, has elucidated a new mechanism by which immune cell macrophages transform into dendritic cell (DC)-like cells. This study revealed that the extracellular matrix component laminin α2 chain and its receptor integrin α7 play an important role in the differentiation process of macrophages into DC-like cells. It has been confirmed that when integrin α7 binding is inhibited, macrophages are particularly active in transforming into DC cell-like cells. This discovery may lead to a new approach to tumor treatment targeting integrin α7, and is expected to pave the way in cancer treatment and immunotherapy.
Research Background
In human bodies, cells need substances that act as scaffoldings to function properly. One of the scaffoldings is a protein called laminin, and currently 19 types are found in human bodies. Among these, the type with a chain called laminin α2 is particularly common in nerves and muscles.
In addition, cells called macrophages, which function as immune cells in the body, can change their properties depending on the surrounding environment in order to prevent disease and heal wounds. It has been thought that these macrophages also function using laminin as a scaffold, but the exact relationship between them has not been fully understood.
Research Contents
This study clarified that by eliminating the function of integrin α7, the receptor that binds most strongly to the laminin α2 chain, a type of immune cell macrophages acquire DC cell-like properties. Dendritic cells act as command centers that present antigens taken in from foreign substances on their cell surface and transmit information to other immune cells such as T cells.
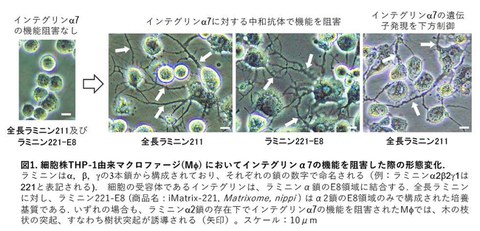
In the experiments, when the activity of integrin α7 was inhibited on a culture plate coated with laminin α2, the spherical macrophages began to extend branch-like protrusions and took on a DC-like morphology (Fig. 1).
Fig. 1 Morphological changes in macrophages derived from the THP-1 cell line upon inhibition of integrin α7 function
The research group confirmed that these cells have active phagocytic activity, express many genes and molecules specific to dendritic cells, and also have the ability to activate T cells. It was also revealed that the intracellular signaling pathways PI3K and AKT are involved in this change.
Furthermore, it is known that monocytes isolated from peripheral blood normally differentiate into macrophages upon stimulation with the cytokine called GM-CSF, and further differentiate into dendritic cells upon the addition of IL-4. However, it was found that when monocytes were cultured on plates coated with laminin α2, they differentiated into DC-like cells without the addition of IL-4 (Fig. 2).
Fig. 2 Differences in cell differentiation depending on the cell culture medium when peripheral blood CD14+ monocytes are stimulated with granulocyte macrophage colony-stimulating factor (GM-CSF)
These results indicate that while immunomodulatory substances known as cytokines have previously been thought to play a central role in dendritic cell differentiation, the cell adhesion environment, including laminin α2 and integrin α7, is also an important factor.
Integrin α7 is also attracting attention as a target for cancer treatment, and the findings of this research are expected to be applied to cancer immunotherapy and other fields in the future.
Research Results
Functional inhibition of integrin α7 resulted in macrophages adopting a DC-like morphology, increasing the expression of DC markers and costimulatory molecules, and acquiring the ability to induce T cell proliferation.
Furthermore, signaling changes via the PI3K-AKT pathway were also confirmed, demonstrating the molecular mechanism by which differentiation is controlled by cell adhesion. This suggests that integrin α7 could be a new target for controlling immune cell function and could be used as a method for inducing dendritic cells in cancer immunotherapy.
Future Developments
This study revealed that the interaction between integrin α7 and laminin α2 chain is a novel pathway that induces DC-like differentiation of macrophages. Inhibiting the function of integrin α7 may promote this differentiation, and the pathway is expected to be utilized in technological development for manipulating immune cells in the future. In particular, the development of antibody therapy or small-molecule inhibitors targeting integrin α7 may be used for DC vaccines and the induction of antitumor immunity in cancer immunotherapy. Furthermore, by designing cell culture medium and biomaterials using laminin α2, it is expected to build a new immunotherapy platform that will increase the induction efficiency of dendritic cells. It is also expected that the expression and function of integrin α7 may be used as a diagnostic marker and a predictor of treatment efficacy.
Notes
The article, “Loss of integrin alpha7-mediated signaling induces a dendritic cell-like phenotype in macrophages cultured on laminin-211/221 isoforms,” was published in Journal of Biological Chemistry at DOI: https://doi.org/10.1016/j.jbc.2025.110419.
