Elucidated the mechanism of antibody dynamics control by glycans
Molecular meridians will pave the way to design antibody drugs
Our bodies have an immune system that protects us from pathogens, and human immunoglobulin G (IgG) is the antibody which plays its central role. IgG not only recognizes and binds to specific antigens but also induces various immune responses through interactions with effector molecules such as Fc receptors (FcR), and complement. In this study, the researchers elucidated the mechanism via computational and experimental approaches by which glycosylation attached to the Fc region of IgG controls the dynamic structural changes of IgG, thereby regulating immune function. This study particularly focused on the importance of "molecular meridians," which transmit the effects of glycosylation at the molecular level, just like the meridians that run throughout human bodies.
Outlines
A research group consisting of Associate Professor Saeko Yanaka at the Exploratory Research Center on Life and Living Systems (ExCELLS) of National Institutes of Natural Sciences (currently Associate Professor at Institute of Science Tokyo), and Professor Koichi Kato (ExCELLS/Nagoya City University) and others has clarified the atomic-level effect that antibody glycosylation, particularly the addition of galactose, has on the structure and dynamics of antibody molecules..
Research Background
Therapeutic antibodies are used to treat a variety of diseases, including cancer and autoimmune disorders. The efficacy of antibodies is largely determined not only by binding to the target antigen but also by exerting effector functions via the Fc region. Glycosylation of the Fc region is an important factor that regulates the effector function of antibodies, and elucidating its mechanism is thought to lead to the development of more effective antibody drugs.
Research Contents
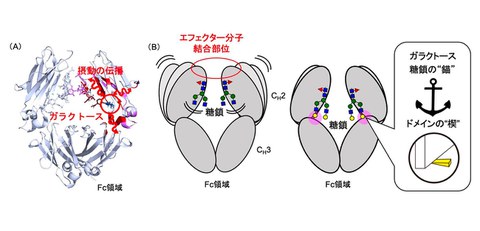
The research group prepared IgG1-Fc with different glycan structures through a technique combining cell engineering and in vitro enzymatic reactions. The researchers analyzed the dynamic structure of the Fc region using stable-isotope-assisted NMR spectroscopy and evaluated the effect of glycosylation on the conformational fluctuations of the Fc region using molecular dynamics simulations. Furthermore, they used dynamic network analysis to identify "molecular meridians". The results of NMR spectroscopy and molecular dynamics simulations revealed that the galactose residues act as an anchor that stops the movement of the glycan and as a wedge that restricts the domain motion. They also discovered that the fucose removal alters the dynamics of amino acid residues involved in binding to specific Fc receptors. These results provide important insights into the atomic-level understanding of the mechanism by which glycosylation controls the dynamic structure of the Fc region of IgG and regulates its effector function. In particular, the existence of “molecular meridians” suggests how the effects of glycosylation propagate throughout the Fc region.
Figure: (A) This is how the structural change at the galactose residue of the glycan is transmitted within the Fc molecule. (B) Galactose residues (yellow circles) act as anchors that stop the movement of glycans and as wedges that restrict the movement of the entire Fc region, facilitating interaction with effector molecules.
Social Impact of the Research
The results of this research will provide a rational design base for optimizing glycosylation of the Fc region in the development of therapeutic antibodies. In the future, by conduction further research into the correlation between glycosylation and antibody structure and function, and by incorporating the new perspective of manipulating "molecular meridians," it is expected to contribute to the development of more effective and safer antibody drugs.
Notes
The article, “Exploring Glycoform-Dependent Dynamic Modulations in Human Immunoglobulin G via Computational and Experimental Approaches,” was published in Proceedings of the National Academy of Sciences of the United States of America at DOI: https://doi.org/10.1073/pnas.2505473122.
