Development of mRNA vaccine indicating therapeutic effect against gastric cancer with peritoneal metastasis
Establishment of new treatments is expected by combining immune checkpoint inhibitors
- Developed a novel mRNA vaccine targeting a protein specific to cancer cells in experimental mouse models.
- By combining mRNA vaccine and immune checkpoint inhibitors, it is indicated that tumors in gastric cancer with peritoneal metastasis are eliminated and confirmed the efficacy in both preventing and treating metastasis.
- In the future, the development of personalized cancer vaccines using mRNA technology is expected to establish immunotherapy for intractable cancers.
Outlines
A research group led by Associate Professor Koji Nagaoka and Professor/Head Kazuhiro Kakimi of the Department of Immunology, Kindai University Faculty of Medicine (Sayama City, Osaka), in collaboration with the University of Tokyo's Research Center for Advanced Science and Technology (Meguro-ku, Tokyo), Institute of Science Tokyo (Chiyoda-ku, Tokyo), the University of Osaka Center for Infectious Disease Education and Research (Suita City, Osaka), Tohoku University Graduate School of Pharmaceutical Sciences (Sendai, Miyagi City), and Hoshi University (Shinagawa-ku, Tokyo), has developed a new vaccine that applies mRNA technology and has demonstrated, for the first time in the world, that when administered to mice in combination with an existing immune checkpoint inhibitor, it exhibits a powerful therapeutic effect against gastric cancer with peritoneal metastasis. If clinical applications to humans progress in the future and lead to the development of personalized cancer vaccines using mRNA technology, it is expected that this will greatly contribute to treating not only gastric cancer but also other intractable cancers.
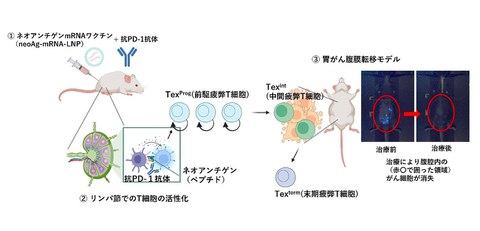
Figure: Treatment of peritoneal metastatic gastric cancer with a combined usage of mRNA vaccine and immune checkpoint inhibitor
Credit: Keiji Itaka
Research Background
Gastric cancer has a high incidence and mortality rate, and even after surgery, it often recurs. If peritoneal metastasis occurs, the median survival time is extremely poor known to be four to six months. Peritoneal metastasis, the most common type of recurrence of gastric cancer, occurs when cancer cells spill over the gastric wall into the abdominal cavity, scattering like seeds of cancer cells, making surgical removal difficult. Chemotherapy is its common treatment, but conventional anticancer drugs and immune checkpoint inhibitors (anti-PD-1 antibodies) have shown limited effectiveness, so the urgent development of new treatments is needed.
In recent years, neoantigens which are antigens specific to cancer cells, are attracting attention as a method for targeting cancer cells and triggering a selective immune response to attack only cancer cells. In previous research in 2021, the research group identified three types of neoantigens from gastric cancer cells, created a vaccine that targeted them, and tested its anti-tumor effects, but the results were insufficient.
Research Contents
The research group created a different type of vaccine to the previous study, targeting neoantigens, and by incorporating a mechanism to efficiently deliver it to cancer cells, they verified its therapeutic effect against gastric cancer with peritoneal metastasis.
First, gastric cancer cells were administered into the abdominal cavity of mice to establish experimental models that reproduced the state of peritoneal metastasis of gastric cancer. Then, the researchers used these models to identify neoantigens and developed mRNA vaccines that target them. It is confirmed that when this vaccine was encapsulated in extremely small capsules called lipid nanoparticles (LNPs) and administered to the mice, it strongly activated killer T cells that target and attack cancer. Furthermore, they found that administering the immune checkpoint inhibitor "anti-PD-1 antibody" in combination with the newly developed vaccine to the mice prevented the spread of cancer cells within the peritoneal cavity and eliminated tumors (indicated in the figure above, red circle at ③).
This research is the first in the world to clarify the combination of an mRNA vaccine and an anti-PD-1 antibody could be a new treatment for peritoneal metastatic gastric cancer, which is resistant to conventional treatments.
The results are expected to establish a new treatment that attacks cancer using mRNA vaccines that target neoantigens. Furthermore, if clinical applications to humans progress in the future and lead to the development of personalized cancer vaccines using mRNA technology, it may be possible to apply this technology in combination with anti-PD-1 antibodies to establish immunotherapy for intractable cancers other than gastric cancer.
Research Details
The neoantigen mRNA vaccine (neoAg-mRNA-LNP) developed by the research group was administered by encapsulating mRNA encoding neoantigens, which are mutated antigens specific to cancer cells, in LNPs. It was found that this vaccine induces the following immune responses and suppresses cancer growth:
1. Antigen presentation and T cell activation
After vaccine administration, the mRNA is taken up by subcutaneous dendritic cells (DCs), where it is translated intracellularly to express neoantigens. This neoantigen was presented via MHC class I molecules and activated
2. T cell infiltration and attack on tumors
Activated CD8⁺ T cells infiltrated the local tumor, recognized and attacked tumor antigens, and destroyed tumor cells.
3. Synergistic effect by combination with anti-PD-1 antibody
When combined with an anti-PD-1 antibody, the differentiation of the following two types of exhausted T cells was promoted within the tumor:
- Texprog cells (precursor exhausted T cells): These cells have the ability to self-renew and are the core cells responsible for maintaining long-term anti-tumor immunity.
- Texint cells (intermediate exhausted T cells): differentiate from Texprog cells and exert anti-tumor effector functions, contributing to tumor elimination.
On the other hand, in the absence of Texprog cells, Texint cells enter a terminal exhausted state in the process of fighting cancer, losing their function, and disappearing. This results in the tumors not being effectively controlled, indicating that the maintenance of Texprog cells is important for sustained tumor immunity.
It has become clear that such activation of the immune system can achieve complete tumor elimination and suppression of recurrence, which could not be achieved with monotherapy. The results of this research are expected to establish a new treatment for peritoneal metastasis of gastric cancer, and by combining neoAg-mRNA-LNP with various chemotherapies, it may be possible to apply it to the treatment of a wide range of cancers.
This research was conducted as part of the "mRNA Drug Discovery Project" promoted by Tatsuhiko Kodama, Professor Emeritus of the University of Tokyo and Project Leader (Specially Appointed Researcher) of the Cancer and Metabolism Project at the University of Tokyo's Research Center for Advanced Science and Technology, under a collaborative research system that makes use of the expertise of each research institution.
The research group used a mouse gastric cancer cell line established by Professor Sachiyo Nomura of Hoshi University, targeting neoantigens identified by Professor/Head Kazuhiro Kakimi and others of the Department of Immunology, Faculty of Medicine, Kindai University. Then the research team led by Professor Keiji Itaka of the Institute of Science Tokyo and the Center for Infectious Diseases Education and Research at the University of Osaka prepared the mRNA, and the research team of Professor Hidetaka Akita of the Graduate School of Pharmaceutical Sciences at Tohoku University was in charge of the lipid nanoparticle formulation. Kindai University has verified the therapeutic effects using a mouse gastric cancer model, and has clarified detailed immunological mechanisms, including the induction of tumor-specific CD8⁺ T cells, analysis of exhausted T cells (Texprog, Texint, and Texterm), and functional evaluation of tumor-infiltrating lymphocytes. This collaboration led to the successful development of a neoantigen-targeting mRNA cancer vaccine formulation, and its efficacy was clearly demonstrated.
Figure: Process for encapsulating mRNA vaccines from neoantigens into LNPs
Credit: Keiji Itaka
Notes
The article, “Neoantigen mRNA vaccines induce progenitor‑exhausted T cells that support anti‑PD‑1 therapy in gastric cancer with peritoneal metastasis,” was published in Gastric Cancer at DOI: https://doi.org/10.1007/s10120-025-01640-8.
