Gut microbiotas influence the immune environment of cancer cells away from the gut via dendritic cells. Identified a new gut microbiota involved in immune checkpoint Inhibitor (ICI).
Published in the British scientific journal "Nature"
- Currently, immune checkpoint inhibitors (ICIs) are used to treat a variety of cancers, but only about 20% of patients achieve long-term therapeutic benefits, and further improvements in treatment outcomes are required.
- It has been reported that the therapeutic effects of ICIs are related to gut microbiota; however, the detailed mechanisms, such as why gut microbiota affect cancers developed in organs other than the intestine (such as the lungs), were unknown.
- In this study, the researchers identified the YB328 strain, a member of the Ruminococcaceae family, as a new gut microbiota involved in the action of ICIs, and further succeeded in culturing it, elucidating its mechanism of action.
- The YB328 strain activates dendritic cells, which are the command center of immune responses in the gut, and these dendritic cells exert their immune effects by migrating to cancer tissue. It has also been shown that further activation of dendritic cells through diversification of the gut microbiota may enhance the effectiveness of ICIs.
- The results of this research suggest that the YB328 strain may enhance the effects of cancer immunotherapy, not only ICIs but also immune cell therapy, and are expected to be applied to next-generation cancer immunotherapy.
Outlines
A research team led by the Division of Cancer Immunology at the National Cancer Center Research Institute (Chuo City, Tokyo; Director: Hiroyuki Mano), has identified the YB328 strain, belonging to the Ruminococcaceae family, as a new gut microbiota that enhances the therapeutic effects of ICIs, and has succeeded in culturing it and elucidating its mechanism of action.
The YB328 strain activates dendritic cells, which are the command center of immune responses in the gut, and these dendritic cells exert their immune effects by migrating to cancer tissue. It has also been shown that further activation of dendritic cells through diversification of the gut microbiota may enhance the effectiveness of ICIs. Furthermore, the YB328 strain was found to be particularly abundant in the guts of patients who responded to the cancer treatment drug PD-1 inhibitor, and it was revealed that this gut microbiota is strongly related to the effectiveness of the treatment and the presence of large numbers of immune cells that attack cancer (PD-1+CD8+ T cells) within the cancer.
This study is the first in the world to visualize and clarify the mechanism by which gut microbiota affects the immune environment of cancers present in organs away from the gut and has elucidated the details of the molecular mechanism that the administration of specific bacteria improves the effectiveness of PD-1 inhibitors.
The results of this study suggest that even in patients for whom ICIs do not provide sufficient benefit, administering the YB328 strain may change the composition of the gut microbiota, creating an environment that is more responsive to ICIs. The YB328 strain has the potential to become a new immunostimulant that enhances the effects of cancer immunotherapy, and future clinical applications are expected.
Research Background
Immune checkpoint inhibitors (ICIs) for cancer have become widely used as one of the standard treatments for various types of cancer. Currently, immune complex therapy, which combines ICIs with other drugs, is also commonly used. However, even with immune complex therapy, most patients do not achieve sufficient therapeutic effects, and the current situation is that only about 20% of cases achieve long-term effects.
One of the important factors that causes differences in therapeutic efficacy is the number of activated CD8+ T cells (especially PD-1+CD8+ T cells) present in the cancer tissue. Recent studies have revealed that the effectiveness of ICIs varies greatly depending on the immune environment of cancer tissue. Specifically, in inflammatory cancers, there is a large accumulation of CD8+ T cells, making ICIs more likely to be effective, whereas in non-inflammatory cancers, there are fewer of these T cells, making ICIs less effective.
Furthermore, the influence of gut microbiota has been attracting attention in recent research. Numerous studies have shown that the diversity of gut microbiota and whether or not certain bacteria are present affect the effectiveness of cancer immunotherapy. However, the mechanism by which gut microbiota affects the immune status of cancer tissues present in organs other than the gut and how they change the abundance of activated CD8+ T cells has not yet been clearly elucidated.
Research Contents
1. Characteristics of gut microbiota involved in the efficacy of ICIs: Increased Ruminococcaceae in successful treatment cases
The research group analyzed the relationship between the effects of cancer immunotherapy (anti-PD-1 antibody) and gut microbiota in a total of 50 patients with non-small cell lung cancer (NSCLC) and gastric cancer. In this study, cancer tissue and stool samples collected before treatment were used to comprehensively analyze immune cells by flow cytometry and evaluated the composition of the gut microbiota by 16S rRNA gene sequence data (Fig. 1a). In particular, the researchers focused on differences in gut microbiota involved in treatment efficacy and the characteristics of T cells infiltrating tumors. They found that bacteria belonging to the family Ruminococcaceae significantly increased in patients who positively responded to treatment. Conversely, the group of patients for whom treatment was ineffective had a significantly higher number of Bacteroidaceae bacteria (Fig. 1b). Furthermore, it was confirmed that patients with a high rate of Ruminococcus colonization had a significantly longer Progression-Free Surviva (PFS) than patients with a low rate, while patients with a high rate of Bacteroidetes colonization tended to have a shorter PFS (Fig. 1c). In addition, an analysis examining the relationship between the gut microbiota and tumor-infiltrating lymphocytes (TILs) revealed that patients with a higher rate of Ruminococcus colonization had a higher frequency of PD-1+CD8+ T cells infiltrating into the tumor (Fig. 1d, e). These results suggest that the composition of the gut microbiota is closely related to the effectiveness of cancer immunotherapy and the immune status within cancer tissue.
Fig. 1
Credit: Shohei Koyama
2. Discovery of a new gut microbiota, YB328, related to the efficacy of ICIs
The research group attempted to isolate and identify Ruminococcaceae bacteria that may be related to immunogenicity from stool samples of patients who responded to and did not respond to anti-PD-1 antibodies. As a result, they were the first in the world to successfully isolate and culture a previously unreported novel bacterial strain YB328, from the stool of a patient who responded (Fig. 2a). By using electron microscopic observation, they also confirmed that the YB328 strain secreted numerous membrane vesicles, suggesting its possible relation in immune activation. In addition, Phocaeicola vulgatus (P. vulgatus) was isolated from the stool of non-responders, and this new bacterial strain was used as a comparison for subsequent experiments (Fig. 2b, 2c). To investigate the combined effect with anti-PD-1 antibodies, the YB328 strain and anti-PD-1 antibodies were administered in combination to SPF mice whose gut microbiota had been eliminated with antibiotics. Tumor shrinkage was observed only in the group administered with the YB328 strain (Fig. 2d). Furthermore, mice administered the YB328 strain showed a significant increase in PD-1+CD8+ T cells in the tract (Fig. 2e), and increased expression of dendritic cell maturation markers (CD86 and MHC-I) was also confirmed (Fig. 2f). Furthermore, when the researchers compared the therapeutic effects of administering the YB328 strain or P. vulgatus with feces from non-responders, therapeutic effect was observed in mice administered with the YB328 strain, even when feces from non-responders were administered at the same time (Fig. 2g). Additionally, T cell receptor (TCR) repertoire analysis revealed that a more diverse TCR repertoire was induced in the YB328-treated group (Fig. 2h).
These results suggest that the YB328 strain may enhance anti-tumor immunity by promoting the infiltration of PD-1+CD8+ T cells through the activation of dendritic cells and changing the intestinal environment to one favorable for immune responses.
Fig. 2
Credit: Shohei Koyama
3. YB328 strain promotes the differentiation and activation of dendritic cells and enhances T cell responses
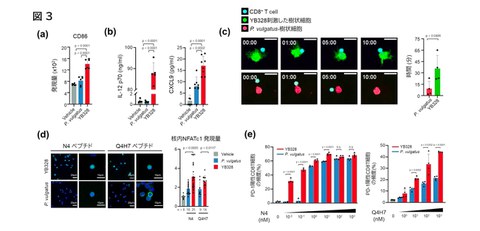
The previous results suggested that the YB328 strain may activate dendritic cells, so the research group conducted experiments using bone-marrow-derived DCs (BMDCs) to elucidate the detailed mechanisms underlying the innate immune response. When the immune responses were compared between stimulation with the YB328 strain or P. vulgatus, it was confirmed that the expression of CD86, a DC maturation marker, was particularly elevated when stimulated with the YB328 strain (Fig. 3a). Furthermore, YB328 stimulation significantly enhanced the production of IL-12p70 and CXCL9, cytokines that promote antigen presentation and T cell migration (Fig. 3b). Also, when BMDCs stimulated with YB328 or P. vulgatus were co-cultured with OVA (ovalbumin) and CD8+ T cells derived from OT-I mice, BMDCs stimulated with YB328 formed more dendrites and maintained interactions with OVA-specific T cells for a longer period (Fig. 3c). In addition, in experiments where stimulation conditions were changed using OVA peptides with different affinities for TCR (high affinity: N4, low affinity: Q4H7), the high-affinity peptide induced nuclear translocation of the transcription factor NFATc1, which is related to T cell activaion, and induced PD-1+CD8+ T cells even at very low concentrations under stimulation with the YB328 strain, whereas no similar response was observed in P. vulgatus (Fig. 3d, e).
On the other hand, when low-affinity peptides were used, it was also shown that the nuclear translocation of NFATc1 and the induction of PD-1+CD8+ T cells became stronger with increasing peptide concentration (Fig. 3d, e).
These results demonstrate that the YB328 strain has the ability to highly efficiently activate dendritic cells, which are innate immune cells, and enhance both the quality and quantity of T cell responses.
Fig. 3
Credit: Shohei Koyama
4. Elucidated the fact that YB328 strain induces and activates CD103+ dendritic cells (DCs), and Toll-like receptor (TLR) signaling is essential for its antitumor effect
To clarify why bone-marrow-derived DCs (BMDCs) stimulated with YB328 induced significantly stronger CD8+ T cell responses than P. vulgatus, the research team performed comprehensive gene expression analysis using RNA sequencing. As a result, it was revealed that the expression of specific transcription factors involved in the differentiation and induction of conventional type 1 dendritic cells (cDC1; CD103+ DCs), such as Irf8 and Batf3, was significantly increased in the YB328 stimulation group (Fig. 4a).
This cDC1 is known to be related to the activation of CD8+ T cells, particularly through cross-presentation.
These changes were also confirmed by protein expression (Fig. 4b, 4c) and progenitor cell induction (Fig. 4d). Furthermore, to verify the extent to which CD103+ DCs contribute to the antitumor effect of the YB328 strain, a similar in vivo treatment experiment was performed using Batf3−/− mice (lacking CD103+ DCs), and it was found that the antitumor effect of the YB328 strain was completely abolished (Fig. 4e). Next, the research group focused on the gene group that was particularly highly expressed in dendritic cells activated by the YB328 strain, and confirmed that the expression of multiple TLRs was increased (Fig. 4f). This suggests that TLR signals play an important role in the activation of CD103+ DCs.
Then the researchers performed similar stimulation experiments using Myd88−/− mice (a major TLR signaling factor) and TLR7/TLR9 double knock-out mice. They found that in both models, induction of CD103+ DCs was abolished, and the antitumor effect of the YB328 strain was completely lost (Fig. 4g–j).
Furthermore, when they performed treatment experiments using multiple TLR ligands (e.g., TLR7/9) instead of the YB328 strain, it is confirmed that similar therapeutic effects were obtained as with the YB328 strain (Fig. 4k).
These results demonstrate that the induction and activation of CD103+ DCs is essential for the expression of the antitumor effect of the YB328 strain, and that the TLR signaling pathway plays a central role in this.
Fig. 4
Credit: Shohei Koyama
5. Visualization of the systemic distribution and dynamics of CD103+ DCs induced by YB328 strain
Based on the results obtained so far, CD103+ DCs have been identified as the cells at the core of the therapeutic effects of the YB328 strain. The research team therefore used a mouse model to analyze lymphatic tissues throughout the body (lamina propria, Peyer's patches, regional lymph nodes, and tumors) to evaluate how CD103+ DCs are induced and activated in the body upon administration of the YB328 strain.
As a result, in mice treated with the YB328 strain, a significant increase in infiltration of CD103+ DCs was confirmed in the lamina propria (Fig. 5a), Peyer's patches (Fig. 5b), regional lymph nodes (dLN) (Fig. 5c), and tumors (Fig. 5d), while no increase in regional myeloid cells was observed in any tissue.
Furthermore, to visually clarify why orally administered YB328 strain increased CD103+ DCs in tumors and regional lymph nodes distant from the gut, we conducted experiments using Kikume Green—Red (KikGR) mice, in which the fluorescent color of cells changes from green (G) to red (R) upon local UV irradiation (Fig. 5e). Mice underwent surgery under anesthesia, and after UV irradiation of the guts only, the YB328 strain was orally administered as before. The tumor treatment effect was then evaluated, and it was confirmed that CD103+ DCs (KikR⁺), which changed color from green to red, were significantly increased in the regional lymph nodes (Fig. 5f, g) and within the tumor (Fig. 5h).
These findings clearly demonstrate that YB328 strain induces activated CD103+ DCs in the gut, which then migrate to lymphoid tissues and tumor parts, enhancing anti-tumor immune responses.
Fig. 5
Credit: Shohei Koyama
6. Validating findings from mice in human tumor tissue: Correlation between YB328 strain carriage rate and immune cell infiltration confirmed
Finally, to verify the findings obtained from functional analysis using mouse models with clinical specimens from actual cancer patients, we performed multiplex immunofluorescence staining analysis on tumor tissue (FFPE specimens) from this cohort before the start of treatment. CD103+ DCs, which are important for the induction of anti-tumor immunity in mice, were identified in human tissues as CLEC9A+ DCs and IRF8-positive DCs. An integrated analysis of the gut microbiota profile and the distribution of tumor-infiltrating PD-1+CD8+ T cells and CLEC9A+IRF8+ DCs was performed on these human tumor tissues. It was revealed that patients with a high rate of YB328 strain carriage had significantly higher levels of tumor-infiltrating PD-1+CD8+ T cells and CLEC9A+IRF8+ DCs (Fig. 6a, b).
These results indicate that the YB328 strain activates dendritic cells in the gut, and the anti-tumor immune response induced by this is reproduced in actual human tumor tissue.
Fig. 6
Credit: Shohei Koyama
This demonstrated that effective T cell responses could be induced even against tumors with low antigenicity. Furthermore, it was confirmed that administration of the YB328 strain enhanced TLR expression in CD103+ DCs, increasing sensitivity to gut microbiota other than the YB328 strain, and that the YB328 strain itself improved the diversity of the gut microbiota.
Future Developments
These results revealed the mechanism by which orally administered gut microbiota exert an immune effect on cancer tissues located far from the gut. Furthermore, it has been shown that it may be possible to induce CD8+ T cells with higher reactivity even in non-inflammatory cancers.
Also, it was suggested that even in patients for whom ICIs are ineffective, administration of the YB328 strain may change the composition of the gut microbiota, potentially creating an intestinal environment that is more responsive to ICIs. Analysis of publicly available data has confirmed that the YB328 strain is naturally carried by approximately 20% of Japanese people, suggesting that it is a safe bacterium.
Based on these results, the YB328 strain is expected to be applied to next-generation cancer immunotherapy as a new oral adjuvant (immunostimulant) that enhances cancer immune responses, with a detailed mechanism of action supported.
Notes
The article, “Microbiota-driven antitumour immunity mediated by dendritic cell migration,” was published in Nature at DOI: 10.1038/s41586-025-09249-8.
