Elucidated the mechanism of astrocyte regeneration destroyed by neuromyelitis optica (NMO)
It is expected to develop new treatments for neurological disorders
- Using a new rat model that reproduces a wide range of realistic lesions, the regeneration mechanism of important glial cells (astrocytes) in the spinal cord destroyed by neuromyelitis optica (NMO) has been elucidated.
- The reason for the persistent damage in NMO is the insufficient regeneration of destroyed astrocytes, but it has been impossible to have detailed observation of the astrocyte regeneration process by using conventional NMO rat models.
- The research group examined the astrocyte regeneration process using a newly developed rat model and
- discovered that fibroblast growth factor 8 (FGF8) can promote astrocyte regeneration. They also found that this treatment increased the neurotrophic factor GDNF and promoted neuronal circuit reorganization, thereby improving paralysis.
- Development of new drugs for various neurological disorders, including cerebral infarction, spinal cord injury, and neurodegenerative diseases, which cause destruction of astrocytes will be expected.
Outlines
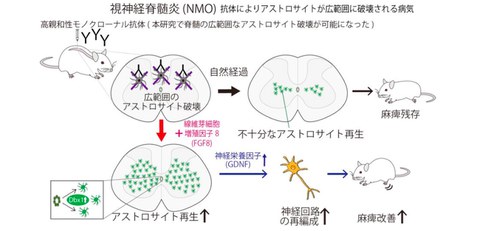
A research group consisting of Specially Appointed Professor Takahide Itokazu (Full Time) at Departments of Molecular Neuroscience and Neuro-Medical Science of Graduate School of Medicine, and Professor Toshihide Yamashita at Departments of Molecular Neuroscience and Neuro-Medical Science, Graduate School of Frontier Biosciences and Immunology Frontier Research Center, and Yoshifumi Ashikawa at the Department of Molecular Neuroscience (ex-student of doctor course) of the University of Osaka, has found the mechanism by which astrocytes regenerate in the spinal cord destroyed by an intractable disease of neuromyelitis optica (NMO), and discovered that FGF8 factor promotes this regeneration. It is shown in rat and cell-based experiments that an increase in glial cell line-derived neurotrophic factor (GDNF), an astrocyte-derived neurotrophic factor, promoted the reorganization of neural circuits and improved paralysis (Fig. 1).
Neuromyelitis optica (NMO) is a disease that causes severe paralysis, numbness, urinary problems, and other symptoms, significantly worsening patients’ quality of life. This condition occurs when astrocytes, which provide nutrients to nerve cells, are destroyed. Astrocytes are crucial for maintaining neuronal function and sustained widespread astrocyte loss poses a major obstacle to recovery from disease. Although a lot of research has been done so far, it remains unclear how astrocytes regenerate after being destroyed in the spinal cord after the onset of NMO.
The research group developed a NMO rat model that exhibits myelitis closely resembles that seen in patients’ conditions and examined the dynamics of astrocyte regeneration after widespread destruction. The results revealed that astrocytes are replenished radially from a region called the central canal at the center of the spinal cord. However, this replenishment is insufficient and takes time, and if left untreated, astrocyte loss will continue for a long period of time. The researchers therefore searched for ways to promote astrocyte replenishment and found that administering FGF8 factor significantly promoted regeneration. This led to an increase in astrocyte-derived neurotrophic factors, which promoted the reorganization of neural circuits and ultimately improved paralysis.
The research results demonstrate that paralysis can be improved by promoting the regeneration of lost astrocytes, opening the possibility of developing new therapeutic strategies for NMO. Furthermore, because astrocyte destruction also occurs in various neurological disorders such as cerebral infarction, spinal cord injury, and neurodegenerative diseases, this research may lead to the development of new drugs for treating these neurological diseases.
Fig.1 Overview
Credit: Takahide Itokazu
Research Background
NMO is caused by widespread destruction of astrocytes due to autoantibodies produced by immune abnormalities, and causes severe paralysis, numbness, urinary problems, and other symptoms. The disease repeatedly relapses and remissions, leading to increasing damage. In recent years, advances in molecular targeted drug therapy that suppresses abnormalities related to recurrence. This made it possible to reduce the recurrence rate, but the damage that occurs during the first recurrence or each recurrence is severe, and in many cases the damage cannot be completely cured.
One possible reason for the incomplete recovery from the damage after the onset of NMO is the insufficient regeneration of destroyed astrocytes. However, in previous rat models, it was difficult to develop the large lesions seen in patients, making it difficult to observe the process of astrocyte regeneration, and therefore this regenerative mechanism had not been researched much so far.
Furthermore, astrocytes present in the spinal cord can be divided into several groups. Each group of astrocytes is generated during development under the control of different transcription factors, and each group has a specific location to exist (Haim et al. Nat Rev Neurosci 2017). Interestingly, it has been reported that even if astrocytes in one area are destroyed, astrocytes in another area do not compensate for this (Tsai et al. Science 2012). It is thought that this mechanism may limit astrocyte regeneration and prevent improvement of paralysis, but it was unclear how astrocytes regenerate when they are destroyed in a wide area.
Research Contents
By developing a rat model that produces widespread spinal cord lesions similar to the pathological condition of patients, the research group established a method for observing the pathological condition after destruction of astrocyte (Fig. 2).
This study revealed that astrocyte regeneration begins after the onset of NMO, spreading radially from around the central canal of the spinal cord. However, this regeneration is insufficient and takes time, and they found that astrocyte loss persists during the critical period for functional recovery.
Therefore, the researchers thought that by promoting astrocyte regeneration, an environment beneficial to functional recovery might be created, and conducted compound screening to search for factors that could promote astrocyte regeneration and identified FGF signaling. In fact, inhibiting FGF signaling in NMO rats suppressed astrocyte regeneration. Conversely, the research group discovered that administration of FGF, especially FGF8, significantly promoted astrocyte regeneration (Fig. 3). They also identified that this regeneration promotion is mediated by the transcription factor Dbx1, which is involved in the distribution of astrocytes in the intermediate spinal cord.
Furthermore, it is clarified by conducting tracer experiments that enhancing FGF8-Dbx1 signaling expands the area of astrocyte regeneration and these astrocytes secrete the neurotrophic factor GDNF, which promotes the reorganization of neural circuits in the corticospinal tract involved in movement. They also revealed in behavioral experiments using multiple species that this reorganization of neural circuits correlated with the improvement of paralysis.
These results suggest that FGF8-Dbx1 signaling is involved in the regeneration of astrocytes after their loss, and by enhancing this pathway, astrocyte regeneration is promoted (Fig. 1), potentially reorganizing neural circuits and improving paralysis.
Fig. 2 Astrocyte loss and regeneration in the NMO model
Credit: Takahide Itokazu
Fig. 3 FGF8 administration promotes astrocyte regeneration
Credit: Takahide Itokazu
Social Impact of the Research
The results of this research indicate that a new treatment strategy of improving neurological disorders by promoting astrocyte regeneration may be effective in NMO pathology, which involves widespread destruction of astrocytes. The experimental method established in this study makes it possible to examine the dynamics of astrocyte regeneration after its loss, which has been difficult to observe until now. It is expected that this method will become an effective tool to elucidate the mechanism behind this phenomenon and discover new treatments.
Notes
The article, “Astrocyte regeneration via FGF8-DBX1 signalling facilitates recovery in neuromyelitis optica rats,” was published in British scientific journal of Brain at DOI: https://doi.org/10.1093/brain/awaf148.
