Reconstruction of human liver sinusoidal blood vessels from iPS cells
Improving bleeding symptoms in hemophilia by creating organoids with enhanced coagulation factor secretion capacity
Key Findings
- Using the unique inverted multilayered air–liquid (IMALI) culture technology that combines a multilayered gel and an air-liquid interface, the research group has successfully reproduced liver's blood vessels, called sinusoids, in three dimensions from human iPS cells in vitro for the first time in the world.
- Through the interaction of four types of precursor cells induced from iPS cells (hepatic endoderm cells, mesenchymal cells, arterial endothelial cells, and liver sinusoidal endothelial progenitor (iLSEP)), the maturation of hepatocytes and the formation of vascular structures including sinusoids proceed simultaneously.
- The liver organoids created using this method secrete high levels of coagulation factors such as factor VIII (FVIII), and transplantation experiments into hemophilia A model mice demonstrated long-term improvement in bleeding symptoms.
- These liver organoids, which have both a sinusoidal structure and metabolic function, are expected to have a wide range of applications, including disease research, drug creation, and regenerative medicine.
Outlines
A research team led by Project Junior Associate Professor Norikazu Saeki at the Human Biology Research Unit of the Institute of Integrated Research (IIR) at Graduate School of Science Tokyo, and Professor Takanori Takebe who holds concurrent positions at Graduate School of Medicine at The University of Osaka and Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe) as part of the Takeda-CiRA Joint (T-CiRA) Program, has succeeded in creating liver organoids (HLBOs) in vitro that contain sinusoids, a blood vessel unique to the human liver, from human induced pluripotent stem cells (iPS cells).
By using a newly developed unique culture method based on the air-liquid interface culture, IMALI method, along with four types of progenitor cells, including hepatic endoderm cells differentiated from iPS cells and liver sinusoidal endothelial cells (iLSEP), they have succeeded in reconstructing the three-dimensional vascular structure unique to the liver. This demonstrated that the maturation and metabolic function of liver cells within the organoids was enhanced, allowing them to efficiently secrete blood coagulation factors. Furthermore, when these liver organoids were transplanted into a hemophilia A model mouse, it was confirmed that bleeding symptoms were improved.
This technology is a fundamental cell manipulation technique for introducing vascular structures specific to human organs into organoids, and is expected to become a tool that to understand biological phenomena and diseases of the human body, many of which are still unexplained. Furthermore, applying liver organoids with enhanced functions to the development of regenerative medicine may lead to innovative therapeutic concepts for blood coagulation disorders and end-stage liver failure, etc.
This result was published in the journal Nature Biomedical Engineering on June 25th (UK time).
Research Background
The liver is an organ that performs a variety of vital functions, including detoxification, nutritional metabolism, production of bile and blood coagulation factors. Among these, unique blood vessels called hepatic sinusoids have extremely thin walls and a fenestrated structure with many tiny holes, allowing efficient exchange of materials between blood and liver cells. Furthermore, sinusoidal blood vessels are thought to be involved in various functions, including immunity and blood coagulation, and are known to be a main source of cells that produce coagulation factors (factors involved in hemostasis) such as factor VIII (FVIII).
On the other hand, hemophilia A is an example of a disease caused by abnormal function of the sinusoidal blood vessels. In hemophilia A, a genetic deficiency of FVIII prevents blood from coagulating, causing severe bleeding even after minor trauma. Currently, replacement therapy, which involves regular injections of coagulating factor preparations, is considered the standard treatment, but it has challenges such as high cost, the burden of administering the drug, and immune reactions. Given this background, there has been a strong demand for the development of regenerative medicine technologies that can sustainably produce coagulating factors in the body.
In recent years, a technology that uses human stem cells such as iPS cells to create tissues that mimic the microstructure of organs, known as organoids, has been attracting attention. Professor Takebe's group also succeeded in creating liver organoids with a vessel network in 2013, but only formed arterial blood vessels, and were unable to create an organ-specific vessel network structure such as sinusoids. Due to these technical limitations, it has been an issue up until now that the functions of the liver, including coagulation factors, have not been fully reproduced. Due to these technical limitations, there has been challenges to fully reproduce liver functions, including coagulation factors.
Research Contents
The research group first established a protocol to efficiently induce iLSEPs from human iPS cells. These cells reproduce the characteristics of endothelium derived from fetal organs and are capable of producing FVIII.
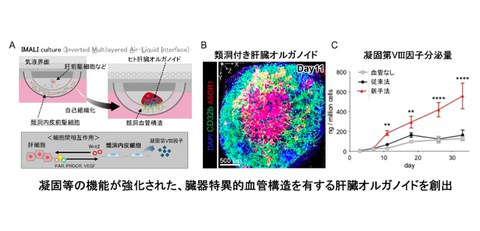
In addition to iLSEP, the research group developed a new three-dimensional culture method (inverted multilayered air–liquid Interface (IMALI) technology in which hepatic endoderm cells, mesenchymal cells, and arterial endothelial cells, which were similarly induced from iPS cells, were combined and encapsulated in a gel, and a three-dimensional structure was constructed using air-liquid interface culture (Fig. 1 A).
This method creates a culture environment where oxygen and nutrients can be supplied from above and below, allowing the cells to naturally gather across the gel boundaries while influencing each other, promoting self-organization.
Fig. 1
A. Overview of the liver organoid generation technology and functions involving sinusoidal endothelial cells found in this research
B. Fluorescence image of the 3D structure of ASGR1-positive hepatocytes (red), CD32B-positive sinusoidal endothelial cells (green), and cell nuclei (blue) that compose liver organoids
C. Amount of coagulation FVIII secreted into the culture medium of liver organoids
As a result, dome-shaped liver organoids (HLBOs) measuring approximately 3 mm in diameter were formed. In these HLBOs, CD32b-positive sinusoidal endothelial cells and ASGR1-positive hepatocytes coexisted, forming a branched vessel network (Fig. 1 B). Single-cell RNA sequencing analysis confirmed that HLBOs contain four types of endothelial cells, including arterial-like, venous-like, and sinusoidal-like, and that CD32b- and LYVE1-positive sinusoidal endothelial cells differentiate predominantly over time. In this process, WNT2 secreted by iLSEP played a central role in promoting hepatocyte maturation and angiogenesis (Fig. 1 A).
In the HLBO formed in this way, the expression of metabolic enzymes and complement specific to the liver was increased, and many blood coagulation factors were secreted. Notably, the FVIII secretion capacity was significantly improved compared to conventional vascularized liver organoids and lasted for over 30 days (Fig. 1 C).
Furthermore, by utilizing the coagulation function of this enhanced "mini-organ," the researchers verified its hemostatic therapeutic effect against coagulation disorders through transplantation experiments using a mouse model of hemophilia A (Fig. 2 A). In the HLBO-transplanted mice, secretion of human FVIII was sustained for up to five months, demonstrating long-term improvement in bleeding symptoms (Fig. 2 B).
Fig. 2
A. Schematic diagram of the experiment verifying the therapeutic effect of liver organoid transplantation on hemophilia A
B. Implementation status of the bleeding test and comparison results of the amount of bleeding
Social Impact of this Research Result
This research is the first in the world to establish a technology for creating liver organoids with organ-specific vessel networks, and also presents a new treatment for blood coagulation disorders, particularly hemophilia A. By constructing and transplanting functional liver organoids using iPS cells generated from the patient's own cells as a base, it is expected to realize a more sustainable treatment that is more sustainable, less burdensome for the patient, and does not rely on external coagulation factor replacement.
In addition, the coagulation factors obtained from liver organoids can be used as medication, and their application in pharmaceutical technology is also within consideration. Furthermore, HLBO is also useful for evaluating drug responses and elucidating the pathology of liver diseases, and is highly valuable as a research platform for drug creation and personalized medicine.
Establishing the technology to create organoids that possess organ-specific vascular structure and functions, which has been difficult to reproduce until now, is expected to be an important milestone toward the regeneration of various organs in the future.
Future Developments
In the future, liver organoids with sinusoids will be used to evaluate drug toxicity, analyze drug dynamics, and apply them to disease research, as well as to evaluate long-term stability and safety, in order to advance practical research aimed at application to humans.
As a fundamental technology for reconstructing organ-specific vessel structures, this technology is expected to be applied not only to liver diseases but also to other organs. In addition, by standardizing and scaling up the manufacturing process, the research group plans to accelerate a wide range of applications, including regenerative medicine, disease models, and drug creation, while also utilizing AI and robotics.
Notes
The article, “Self-organization of sinusoidal vessels in pluripotent stem cell-derived human liver bud organoids ,” was published in Nature Biomedical Engineering at DOI: 10.1038/s41551-025-01416-6 .
Technical Glossary
- Takeda-CiRA Joint Program
A joint regenerative medicine research project conducted in collaboration between academic researchers at Takeda Pharmaceutical Company Limited, the Center for iPS Cell Research and Application (CiRA) at Kyoto University, and Institute of Science Tokyo.
- Induced pluripotent stem cells (iPS cells)
A type of cell that can be produced from somatic cells such as skin cells. These cells can differentiate into various organs and tissues.
- Sinusoid
A special vascular structure with tiny holes that is unique to the liver. In addition to efficiently exchanging nutrients and oxygen between blood and liver cells, it is also known as a cell that secretes blood coagulation factor.
- Human liver organoid (HLBO)
A small 3D liver tissue formed by self-organization of multiple cells derived from human iPS cells.
- Hepatic endoderm cells
A type of cell originates from the endoderm, which is the progenitor cell of the liver, and has the ability to differentiate into hepatocytes and bile duct cells.
- Liver sinusoidal endothelial progenitor (iLSEP)
These cells are the foundation of the formation of sinusoidal blood vessels in the liver, and are derived from human iPS cells.
- Inverted multilayered air–liquid interface (IMALI) technology
A culture method in which cells are cultured at the interface between air and culture solution, promoting gas exchange and nutrient supply, making it easier for cells to perform their inherent functions.
- Hemophila A
A genetic deficiency of coagulation factor VIII (FVIII) impairs blood coagulation, making people more susceptible to bleeding. It is the most common blood coagulation disorder.
- Mesenchymal cells
These cells can differentiate into supporting tissues, etc., and are involved in structural support and signal transduction within organs.
- Arterial endothelial cells
Cells lining arterial blood vessels that respond to changes in blood flow and blood pressure. In differentiation of vascular endothelial cells derived from iPS cells, high concentrations of vascular endothelial growth factor A (VEGF-A) are generally used, which tends to induce arterial-like endothelial cells.
- Single-cell RNA sequencing analysis
A technology that measures gene expression in individual cells.
- WNT2
It is a type of protein relates to development and tissue regeneration, and is involved in blood vessel formation and cell differentiation induction.
- Complement
A group of proteins involved in the elimination of pathogens and immune responses. Many of which are produced in the liver.
