Discovery of new ECM proteins and synthetic peptides that promote myelination
Myelination by oligodendrocytes is enhanced through LM411 and its derived peptide A4G47
- A research group has discovered that extracellular matrix (ECM) protein laminin (LM)-411 and a synthetic peptide A4G47 derived from its amino acid sequence promote myelination in oligodendrocytes, which form myelin sheaths in the central nervous system.
- The researchers identified laminins expressed in central nervous system tissues during myelination and confirmed specific activity for LM411 and A4G47 through analysis of their recombinant proteins and peptides.
- LM411 and A4G47 are expected to be utilized as new molecular tools for promoting myelination, and have shown potential application in treating diseases such as multiple sclerosis, Alzheimer's disease, depression, and decline in brain function related to aging.
Outlines
A research team led by Associate Professor Nobuharu Suzuki at Graduate School of Medical and Dental Sciences, Institute of Science Tokyo, is attempting to elucidate the control mechanism of myelin function in the central nervous system, which is known as one of the main causes of demyelinating diseases such as multiple sclerosis, as well as decline in brain function related to aging, Alzheimer's disease, and mental illnesses such as depression.
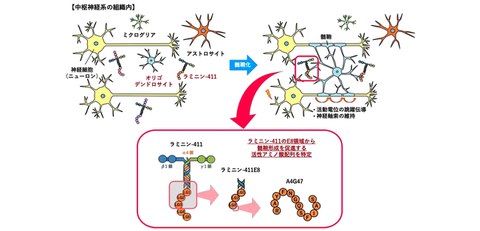
In this study, the research group analyzed laminin, an extracellular matrix (ECM) protein, in collaboration with Professor Motoyoshi Nomizu at Department of Clinical Biopharmacy (at the time), School of Pharmacy, at Tokyo University of Pharmacy and Life Sciences, and Endowed Research Chair Professor Kiyotoshi Sekiguchi at Institute for Protein Research, The University of Osaka. First, among the many LM isoforms, the research group discovered LM111, LM211, and LM411, that are expressed in central nervous system tissues. Next, researchers used these recombinant proteins to examine their effects on oligodendrocytes, which are myelination cells, and found that LM411 significantly promoted myelination (Fig. 1). Furthermore, a similar promoting activity was observed in E8, the region that binds to integrin, the receptor for LM411, and in the synthetic peptide A4G47, which has a cell adhesion amino acid sequence present in this region (Fig. 2).
This is the first discovery of a myelination peptide derived from an ECM protein.
This study has revealed the role of ECM proteins in myelination and LM411 and A4G47 have a potential to be used as myelination tools or myelination promoters and are expected to contribute to the treatment of the aforementioned diseases and to improving their quality of life.
Fig. 1
Oligodendrocytes cultured on LM411. Culturing oligodendrocytes on LM411 significantly promoted the branching of cell processes required for myelin membrane formation
GalC: oligodendrocyte marker, NG2: oligodendrocyte precursor cell, DAPI: cell nucleus (Sasaki et al., 2025,
0.1002/glia.70027)
Credit: Kiyotoshi Sekiguchi
Fig. 2
Overview of research findings. Among the several LM isoforms present in the central nervous system, LM411 promoted myelination of oligodendrocytes. Also, the E8 domain of LM411 and the A4G47 sequence within that domain promoted myelination
Credit: Kiyotoshi Sekiguchi
Research Background
In the central nervous system, the formation of myelin sheaths by oligodendrocytes around nerve axons dramatically improves the conduction velocity of nerve action potentials, allowing the neural network to function normally. Since the myelin sheath also functions to maintain axon homeostasis, demyelinating diseases such as multiple sclerosis ultimately lead to axonal degeneration, resulting in severe neurological symptoms. In recent years, it has been reported that abnormalities in myelin function are the main cause of Alzheimer's disease, mental illnesses such as depression, and even the decline in brain function due to aging, and its importance is gaining attention [References 1].
The molecular mechanisms of myelination have been elucidated in detail, including the functions of myelin proteins, cytoskeletal molecules, and transcription factors. However, despite the rich interstitial volume of central nervous system tissues, the functions of extracellular matrix (ECM) molecules remain relatively poorly understood [References 4].
In terms of applications, ECM molecules do not need to be introduced into cells, so they can be utilized relatively easily for a variety of purposes. Against such background, the research group is conducting research into the functional analysis and application of ECM molecules.
In this study, the researchers focused on laminin, the main ECM protein constituting the basement membrane, and investigated the effects of the numerous laminin isoforms on oligodendrocytes and their myelination activity, as well as attempting to minimize the active part.
Research Contents
Laminin is composed of three types of subunits: α, β, and γ chains. The research group then examined the expression of the five types of α chains (α1-5) in central nervous system tissue during myelination, revealing that α1, α2, and α4 were expressed.
Next, the researchers investigated the effects of recombinant laminin proteins (LM111, -211, and -411) containing these α chains on oligodendrocytes. As a result, LM411 had a significant myelination activity and apoptosis suppression effect. Furthermore, the integrin-binding domain of LM411, laminin-411E8, was also shown to promote myelination.
Finally, the effects of synthetic peptides (12 amino acids) consisting of ten types of cell adhesion amino acid sequences identified in LM411E8 [Reference 5] were examined, and A4G47 (RAYFNGQSFIAS (single-letter representation)) exhibited amino acid sequence-dependent myelination activity.
Social Impact of this Research Result
The identification of the peptide A4G47, which is composed of only 12 amino acids, from the ECM protein LM411 is expected to be applied to various research. Ultimately, this technology may contribute to the improvement of myelin dysfunction in demyelinating diseases such as multiple sclerosis, as well as mental diseases such as Alzheimer's disease, which has been attracting attention in recent years, and depression, and even to the improvement of brain function decline due to aging.
Future Developments
The research group will plan to conduct further research aimed at elucidating the detailed molecular mechanisms of oligodendrocyte myelination mediated by LM411 and A4G47, as well as the application research mentioned above.
Notes
The article, “Myelin Formation by Oligodendrocytes Is Enhanced Through Laminin-411 and Its Derived Peptide,” was published in GLIA at DOI: 10.1002/glia.70027 .
References
[1] Depp et al, 2023, Nature:Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease
[2] Kaya et al, 2023, Nature Neuroscience:CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging
[3] Sasmita et al, 2024, Nature Neuroscience:Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice
[4] Yamada et al, 2022, Frontiers in Cell and Developmental Biology:The Molecular Regulation of Oligodendrocyte Development and CNS Myelination by ECM Proteins
[5] Katagiri et al, 2012, Archives of Biochemistry and Biophysics:Screening of Integrin-Binding Peptides From the Laminin α4 and α5 Chain G Domain Peptide Library
