Developing innovative precision medicine to cut off the nutritional pathway of cancer
New therapeutic strategies by controlling the tumor microenvironment
Key Findings
- A new molecular targeted drug has been developed that suppresses tumor growth by targeting the metabolic pathway of nicotinamide, which is a key component in tumor (cancer) tissue.
- Using University of Osaka's nucleic acid medicine technology, a groundbreaking therapy was designed that uses an artificial template (antisense) to precisely control target genes with pinpoint accuracy. By targeting metabolic pathways, it is possible to suppress the survival and progression of cancer-associated fibroblast (CAFs) in tumor tissue and break the vicious cycle of immune cell exhaustion.
- As a method for rapid and precise control of target molecules within cells, it is expected to be applied to a variety of intractable diseases.
Outlines
A research group consisting of Specially Appointed Assistant Professor Tomoaki Hara , Specially Appointed Assistant Professor Sikun Meng, and Specially Appointed Professor Hideshi Ishii (Medical Data Science) from Graduate School of Medicine at the University of Osaka, in collaboration with the research group led by Professor Satoshi Obika, Assistant Professor Takashi Osawa (Bioorganic Chemistry) from Graduate School of Pharmaceutical Sciences at the University of Osaka, and Project Leader Yuuya Kasahara from Laboratory of XNA Screening and Design, National Institutes of Biomedical Innovation, Health, and Nutrition, has developed a targeted nucleic acid medicine that precisely controls nicotinamide N-methyltransferase (NNMT) , which is highly expressed in cancer-associated fibroblasts (CAFs), as a key to controlling the epigenetic mechanism, one of the typical characteristics of tumor tissue.
The research group applied nucleic acid medicine technology developed at the University of Osaka Graduate School of Medicine to precisely target NNMT in CAF cells using an artificial template (antisense) in preclinical animal experiments, demonstrating that this technology exhibits antitumor effects against intractable cancers.
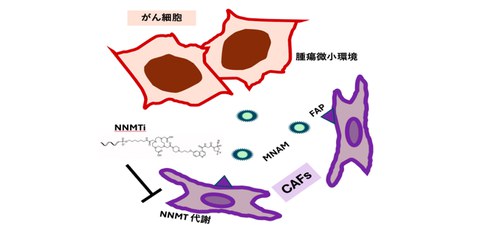
By precisely targeting NNMT, it became possible not only to suppress the survival and progression of CAFs but also to break the vicious cycle of CAF-induced exhaustion of immune cells (Fig. 1).
This research is expected to provide a method for rapid and precise control of target molecules within cells, which can be applied to a variety of intractable diseases.
Fig. 1
Inhibition of NNMT metabolism by NNMTi exerts antitumor effects.
Research Background
Epigenetic mechanisms, a typical feature of cancer, are involved in gene transcription through histone and DNA methylation, translation through RNA methylation, and activation of physiological functions through methylation of other proteins and various molecules.
These methylations are promoted by the biosynthesis of S-adenosyl-l-methionine (SAM) as a single-carbon metabolic pathway. However, in tumor tissues, this overall methylation is abnormally active, and also acts on normal tissues which do not need treatment. Therefore, in order to administer treatment properly, it is important to distinguish between cancer and normal cells.
In addition, methylated nicotinamide (MNAM) produced by NNMT inhibits the production of interferon by immune cells, creating a vicious cycle in which anti-tumor immunity does not function adequately. For this reason, new molecular targeted therapies that can capture the overall tumor microenvironment, and specifically attack the area with pinpoint accuracy are expected to be developed.
Research Contents
In this study, we aimed to precisely target NNMT activated in CAFs by using a specific fibroblast activation protein (FAP) in CAFs as a marker, and to improve the tumor microenvironment with pinpoint accuracy while considering the entire tumor.
The research group aimed to break the vicious cycle of MNAM by inhibiting NNMT in CAFs, reactivating exhausted lymphocytes, and causing tumor regression and preventing cancer recurrence, and it developed a nucleic acid drug that uses an artificial template (antisense) that targets the NNMT gene using FAP as a marker, by screening the sequence and artificially synthesizing nucleic acids.
This artificial template antisense drug has been shown to improve the tumor microenvironment and exhibit good anti-tumor effects in preclinical animal experiments, and it is expected , together with standard cancer treatment drugs, to cure cancer completely.
Social Impact of This Research Result
The research group has developed a precise delivery technology for artificial templated nucleic acid medicines that can accurately target any gene in a specific cell amid complex intercellular interactions. This expanded the possibilities for diagnosis and drug discovery, and established a foundation for the creation of groundbreaking new technologies. These technologies have made it possible to block metabolic pathways that were previously uncontrollable and are therefore fundamental research aimed at curing cancer completely.
Notes
The article, “Antisense oligonucleotide targeting nicotinamide N-methyltransferase exhibits antitumor effects,” was published in American Journal of Molecular Therapy - Nucleic Acids at
DOI: https://doi.org/10.1016/j.omtn.2025.102548.
Links
Technical Glossary
- Artificial template (antisense)
A nucleic acid molecule with enhanced stability due to its non-natural nucleic acid structure. By binding to specific sequences of target mRNAs, it exert an inhibitory effect on gene expression.
- Nicotinamide N-methyltransferase (NNMT)
A metabolic pathway of a type of vitamin B3 that is highly specific to cancer. Methylnicotinamide, the product of this enzyme, is secreted in large quantities from CAFs, causing the cancer immunity of surrounding immune cells to become exhausted.
- S-adenosyl-l-methionine (SAM)
It acts as a methyl group donor and is consumed in the methylation reactions of methyltransferases including DNA, RNA, and proteins, etc. .
- Tumor microenvironment
Tissue formed by tumor cells and the cells and molecules surrounding them. It contains immune cells, fibroblasts, blood vessels, extracellular matrix components such as collagen and fibronectin, cytokines, and various metabolic products, and plays an important role in tumor growth.
