First in-human investigator-initiated clinical trial to launch for refractory prostate cancer patients: Novel alpha therapy targets prostate-specific membrane antigen
A research team at Osaka University will start an investigator-initiated clinical trial for refractory prostate cancer patients after successful development of a new alpha-ray therapeutic agent ([At-211] PSMA-5) and confirmation of its efficacy in animal models. This will be a world-first in-human clinical trial with [At-211] PSMA-5.
Prostate cancer is on the rise worldwide and is the most commonly diagnosed new cancer in men in Japan. Various treatments are offered for prostate cancer, but the prognosis is very poor when the disease is resistant to standard treatment and associated with multiple metastases.
In recent years, theranostics, which involves changing the radionuclide labeled to a compound that binds to a target, has been attracting attention as an integrated approach from cancer diagnostic imaging to therapy. Prostate-Specific Membrane Antigen (PSMA) is also gaining recognition as an innovative target for theranostics, enabling deployment from PET (positron emission tomography)-imaging based lesion detection to targeted radionuclide therapy. Furthermore, alpha irradiation from inside the body via intravenous injection enables treatment of metastases throughout the body.
In addition, astatine (At-211) is a nuclide that emits alpha rays with higher energy than conventional radiation and is expected to be effective even in patients exhibiting beta-ray treatment resistance. Since astatine can be produced using an accelerator, it enables domestic production without the need to import the radionuclide from abroad. A new cyclotron dedicated to its production will be installed at Osaka University with financial support by the Ministry of Economy and Industry, allowing for large-scale astatine supply.
Patients with castration-resistant prostate cancer with multiple metastases are treated with chemotherapy and other therapies. However, this comes with a significant number of side effects, and patients may become refractory to treatment in a short period. On the other hand, targeted radionuclide therapy rarely causes severe side effects, and treatments using short-range alpha radiation do not require hospitalization in a specialized room.
"Astatine can be manufactured domestically using an accelerator, and by establishing a manufacturing base, it is expected that a number of patients will be able to receive the treatment without hospitalization," says Tadashi Watabe, the principal investigator of the study. "In the future, it is expected that astatine will be used as a Japan-originated therapy for prostate cancer patients in need of treatment around the world."
Fig. 1
Targeted alpha therapy using an astatine-labeled PSMA ligand ([At-211]PSMA5)
Credit: Tadashi Watabe (Osaka University)
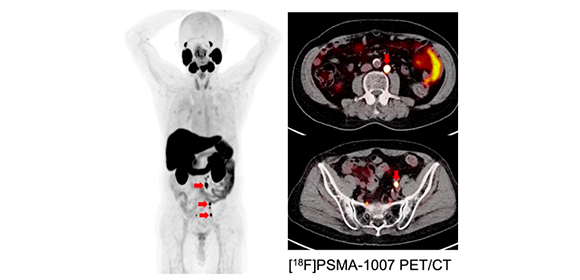
Fig. 2
Companion PET images targeting PSMA: Hormone refractory prostate cancer with multiple lymph node metastases (red arrow)
Credit: Tadashi Watabe (Osaka University)
Fig. 3
Antitumor effect after single administration in prostate cancer model mouse (left) and biodistribution in tumor-bearing model (right): High accumulation in tumor (arrow) can be confirmed.
Credit: Tadashi Watabe (Osaka University)
