Lysosomal exocytosis causes cancerous cells to grow in acid addiction
A group of researchers from Osaka University has clarified that phosphatases of regenerating liver (PRLs) activate release of their ingredients such as condensed protons by stimulating lysosomal exocytosis and allow cancer cells to grow in an acidic environment (acid addiction). PRLs involved in the control of cell growth and migration are highly expressed in advanced tumors and metastases.
Extracellular pH is usually maintained around 7.4 in multicellular organisms and cells are optimized to proliferate under this condition and even tiny changes in extracellular pH have a significant effect. On the other hand, the pH of the extracellular environment of cancer cells, which is as low as 6.5 because of the Warburg Effect (elevated glucose metabolism decreases the pH in the microenvironment due to lactate secretion), promotes cancer metastasis and remodels the tumor microenvironment. This is in striking contrast to the adjacent normal tissue whose pH is strictly maintained around 7.4.
It was known that cancer cells are acidic but not why cancer cells can continue to survive and thrive under harmful conditions in an acidic tumor microenvironment. In this study, the researchers found that PRLs make cells optimized to grow in an acidic environment. They also found that expression of PRL3, which is known to be involved in the metastatic processes of many different human cancers, shifted the optimum pH for proliferation from 7.5 to an acidic 6.5, and that cells expressing PRL3 survived and proliferated in an acidic environment but ceased to proliferate under the standard conditions of around pH 7.4.
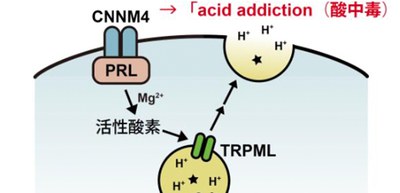
Further experiments clarified the molecular link between PRL and lysosomal exocytosis across species, showing that disruption of transient receptor potential mucolipin (TRPML) in cancer cells abolished PRL-stimulated lysosomal exocytosis, acid addiction, and metastasis. Thus, PRL is the molecular switch that gets cells addicted to acidic conditions, which should benefit cancer cells to thrive in an acidic tumor microenvironment.
In addition, using Genome-scale CRISPR-Cas9 knockout screening and subsequent analyses, the researchers revealed that PRL promotes H+ extrusion and acid addiction by stimulating lysosomal exocytosis and that lysosomal exocytosis mediated by TRPML is important in acid addiction and metastatic tumor formation by PRL.
Hypoxia (low oxygen) and elevated extracellular acidification are prevalent features of solid tumors. Increased oxygen consumption by tumors and impaired delivery of oxygen create hypoxia, which has been actively studied. For example, angiogenesis inhibitor bevacizumab (brand name: Avastin) has already been used as a cancer treatment drug. Bevacizumab works by inhibiting the action of a hypoxia-induced angiogenesis growth factor that binds to receptors on blood vessels. This inhibition reduces microvascular growth of tumor blood vessels and thus limits the blood supply to tumor tissues.
On the other hand, since elevated extracellular acidification has not been studied very much, this group’s achievements will lead to the development of cancer therapy that target this acidity could be of value therapeutically.
Figure 1
Figure 2
The article, “The oncogenic PRL protein causes acid addiction of cells by stimulating lysosomal exocytosis,” was published in Developmental Cell at DOI: https://doi.org/10.1016/j.devcel.2020.08.009.
