Elucidating mechanisms behind catalytic oxidation by synchrotron radiation at the SPring-8
A group of researchers from Tokyo University of Agriculture and Technology, Osaka University, and Kyoto University, has clarified detailed mechanisms of the copper (Cu) cycle in palladium (Pd)/Cu-catalyzed dehydrogenative coupling by utilizing synchrotron radiation on the BL01B1 beamline at SPring-8 of the Japan Synchrotron Radiation Research Institute (JASRI).
Pd/Cu-catalyzed oxidation, a process that oxidizes compounds using the oxygen in air without using solvent, sees widespread industrial use. In particular, Pd/Cu-catalyzed dehydrogenative coupling, which involves the direct generation of C-C bonds by the oxidation of two C-H bonds, is an industrially important process for the production of aromatic polyimide precursors. Cross-dehydrogenative coupling needs both the Pd/Cu catalysts, but the coupling mechanisms were unknown.
Aromatic polyimides, which have excellent properties such as thermal stability, mechanical strength, toughness, and high electrical insulation, are used in the electric, aerospace, and aviation industries.
In this study, this group focused on dehydrogenative arene (aromatic hydrocarbons) coupling of dimethyl phthalate (phen), which is used for the industrial production of aromatic polyimide precursors, and investigated the dehydrogenative coupling of dimethyl phthalate catalyzed by palladium(II) acetate (Pd(OAc)2)/copper(II) acetate (Cu(OAc)2)/phen.
The dehydrogenative coupling of arenes, the most straightforward coupling between arenes, is generally catalyzed by a Pd complex with a Cu cocatalyst in the presence of oxygen. This group clarified the mechanisms for Pd/Cu-catalyzed dehydrogenative coupling of dimethyl phthalate in 2018.
“Mechanistic Insights on Pd/Cu-Catalyzed Dehydrogenative Coupling of Dimethyl Phthalate.”
In this study, the researchers focused on catalytic reactions via Cu complexes in order to fully unveil the mechanisms for dehydrogenative coupling of dimethyl phthalate catalyzed by [Pd(OAc)2]/[Cu(OAc)2]/1,10-phenanthroline·H2O (phen·H2O). By analyzing the XANES (x-ray absorption near edge structure) and the EXAFS (extended x-ray absorption fine structure) of these Cu complexes using synchrotron radiation, which is 10 billion times brighter than the Sun, the researchers revealed the catalytic behavior of the Cu complexes in the Pd/Cu-catalyzed dehydrogenative coupling of dimethyl phthalate as follows:
- During a reaction, dinuclear copper(II) acetate (Cu(OAc)2) converts to a mononuclear divalent [Cu(OAc)2]·2H2O species in catalytic reaction solvent.
- 2 equiv of Cu(OAc)2·H2O oxidizes the zerovalent “Pd(phen)” species to divalent Pd(OAc)2(phen), and the divalent [Cu(OAc)2]2·2AcOH species is regenerated by the treatment of monovalent Cu(OAc) with AcOH in air.
This group also found that a Cu catalyst was indispensable for the catalysis but coordination of the phen ligand to the Cu catalyst significantly inhibited catalysis. Thus, this group recommends utilizing [Pd(OAc)2(phen)] as the Pd catalyst instead of the addition of phen to the [Pd(OAc)2] /Cu(OAc)2 system.
Since Pd/Cu-catalyzed oxidation is used in many oxidation reactions, the development of catalysis based on findings in this study is anticipated. This group’s achievements will lead to the efficient synthesis of aromatic polyimide precursors.
Figure 1
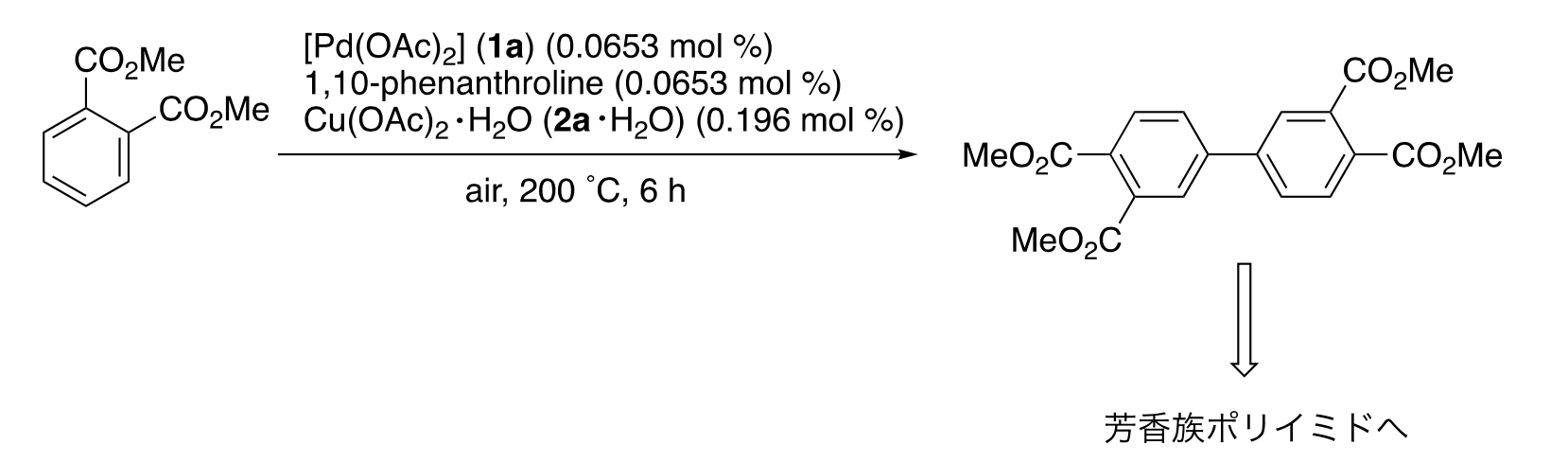
Figure 2
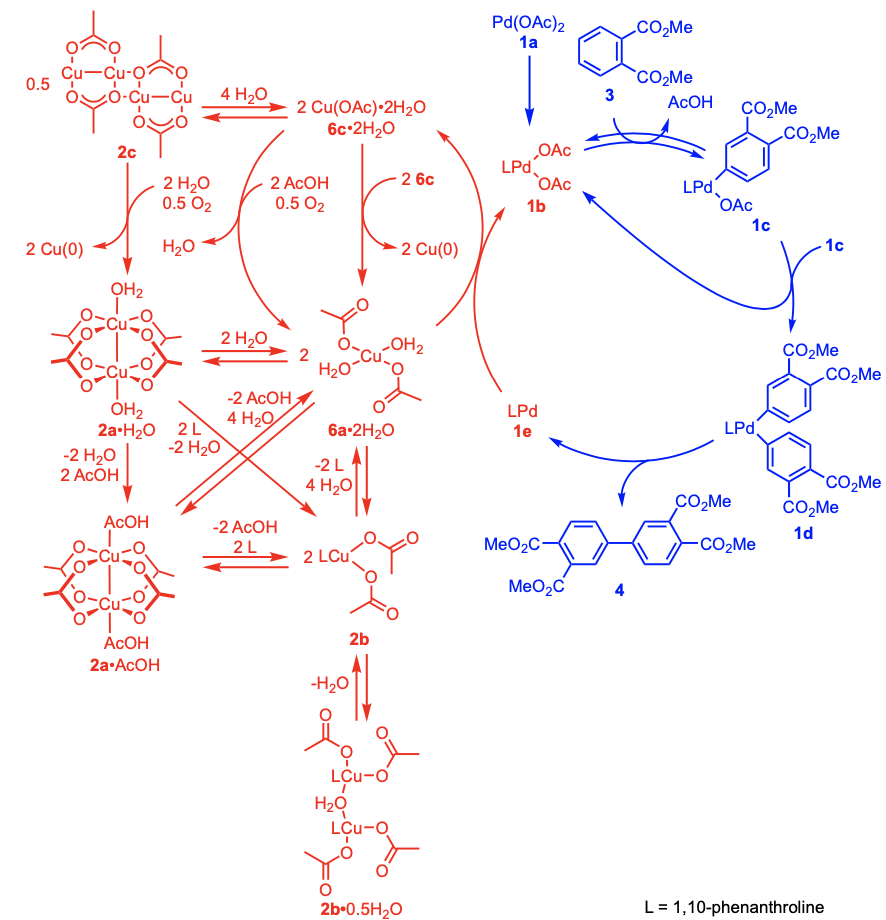
Figure 3
The article, “Pd/Cu-Catalyzed Dehydrogenative Coupling of Dimethyl Phthalate: Synchrotron Radiation Sheds Light on the Cu Cycle Mechanism,” was published in ACS Catalysis at DOI: https://pubs.acs.org/doi/10.1021/acscatal.0c00918.
