How does the extinction of a conditioned taste aversion occur?
Brain mechanisms for cannabinoid-mediated extinction of aversive memories
A group of researchers led by Associate Professor Hiroki Toyoda from the Graduate School of Dentistry of Osaka University has clarified brain mechanisms for the extinction of aversive memories.
The gustatory insular cortex conceives taste intensity and quality and plays a critical role in conditioned taste aversion (CTA) learning. A taste aversion is a tendency to avoid or make negative associations with a food that one ate before getting sick. It is caused after ingestion of the food causes nausea, sickness, or vomiting. This is an example of classical conditioning and is a type of learning in which individuals learn to associate stimuli. However, even if a person associates oysters with getting sick and becomes averse to eating them, the person can become able to eat them again.
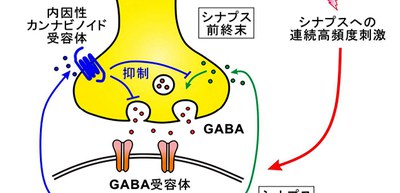
It is thought that this is caused by neural activity to induce CTA memory extinction, but little is known about efficacy of the long-term expression of synaptic changes (the form of synaptic plasticity, such as Long-term potentiation (LTP) and long-term depression (LTD)) at GABAergic synapses in the insular cortex. GABA (gamma-aminobutyric acid) in the central nervous system is the primary inhibitory neurotransmitter, decreases the neuron's action potential.
Brain functions are closely correlated with plasticity at GABAergic synapses (GABAergic synaptic plasticity). LTP and LTD of GABAergic synaptic transmission are factors affecting neuronal synaptic plasticity. It is thought that GABAergic synaptic plasticity is deeply involved in CTA learning, and LTP and LTD are considered to be key synaptic mechanisms involved in learning and memory. However, how GABAergic synaptic plasticity is modulated remains unclear and little is known about long-term changes of synaptic efficacy at GABAergic synapses. Thus, this group examined the synaptic mechanisms of long-term plasticity at GABAergic synapses in layer V pyramidal neurons of the mouse insular cortex.
In this study, the researchers provided evidence that the interaction between endocannabinod (eCB) and nitric oxide (NO) signaling plays a key role in modulating plasticity at GABAergic synapses as follows.
- In response to a prolonged high-frequency stimulation (HFS, 100 Hz, 4 s) to the gustatory insular cortex, GABAergic synapses displayed eCB-mediated LTD.
- NO production was necessary for the CB1R-mediated suppression of eIPSCs (evoked inhibitory postsynaptic currents) in GABAergic synaptic transmission.
- Activation of CB1Rs induced LTD at GABAergic synapses (LTDGABA), while production of NO induced LTP (LTPGABA). In addition, activation of CB1Rs at the presynaptic terminals suppressed the action of NO that increases GABA release.
- In the presence of leptin, a hormone that regulates food intake, energy homeostasis, as well as learning and memory, the prolonged HFS caused LTPGABA, just like when cannabinoid 1 receptors (CB1Rs) were blocked by a CB1R antagonist, the same stimuli caused LTPGABA.
Previous studies showed that eCBs contributed to induction of LTDGABA in the basolateral amygdala and also were involved in amygdala-dependent extinction of aversive memories.
This group’s study results indicate that long-term plasticity at GABAergic synapses in the insular cortex can be modulated by the combined effects of eCB and NO signaling. These forms of GABAergic synaptic plasticity may be crucial synaptic mechanisms in taste learning.
This group’s achievements made a great contribution to the understanding of synaptic mechanisms in taste learning. It is anticipated that the results of this study will develop into applied research for children’s healthy development and preventing adult diseases caused by dietary habits.
The article, “CB1 cannabinoid receptor-mediated plasticity of GABAergic synapses in the mouse insular cortex,” was published in Scientific Reports at DOI: https://www.nature.com/articles/s41598-020-64236-5.
Related links
Department of Oral Physiology, Graduate School of Dentistry, Osaka University (link in Japanese)
