Cryo-electron microscopy clarifies mechanisms behind membrane transport of bacterial toxin
A group of researchers from Kyoto Sangyo University and Osaka University has clarified the structure of the iota toxin produced by Clostridium perfringens type E. They also determined the structures of the translocation channel Ib-pore and its complex with Ia at high resolution (2.8~2.9 angstrom) using the single particle cryo-electron microscopy (EM) technique.
The iota toxin, a binary toxin, comprises two independent polypeptides: an enzymatic component (Ia, an ADP-ribosyltransferase) and a binding component (Ib), which is involved in cell binding and translocation of Ia across the cell membrane.
The diameter of a pore formed by the binding component is so small that structural change is required for translocation of an enzymatic component into the host cell. However, translocational unfolding mechanisms in the binary toxin complex were unknown.
Pro-Ib monomer binds to the lipolysis-stimulated lipoprotein receptor (LSR), the host receptor for the binary toxin, and is activated by proteolysis. This triggers oligomerization to form the prepore and also the Ib-pore as a metastable state.
In this study, the researchers oligomerized the Ib monomer into a heptameric pore by ethanol addition. The oligomerization efficiency of Ib-pore, which was enhanced by ethanol addition, allowed for the structural analysis of the Ib-pore.
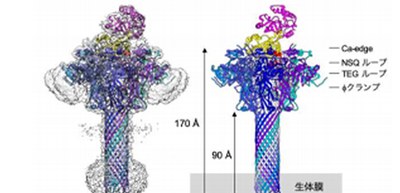
Furthermore, they added Ia at three-fold molar excess prior to their 2nd data set collection, analyzing the structure of the Ia-bound Ib-pore. As a result, the Ib-pore structure demonstrated a structural framework similar to that of the catalytic ϕ-clamp of the anthrax protective antigen pore.
The Ia-bound Ib-pore structure showed a unique binding mode of Ia, one Ia bound to the Ib-pore, and the Ia amino-terminal domain formed multiple weak interactions with two additional Ib-pore constriction sites. Ib-binding induced tilting and partial unfolding of the Ia N-terminal α-helix, permitting its extension to the ϕ-clamp gate and causing protein translocation.
This group’s achievements will lead to the development of drugs that prevent the membrane transport of an enzymatic component into the cytosol.
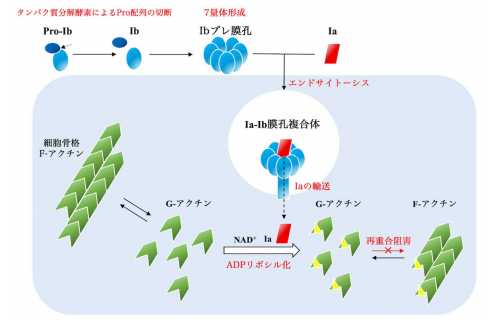
Figure 1
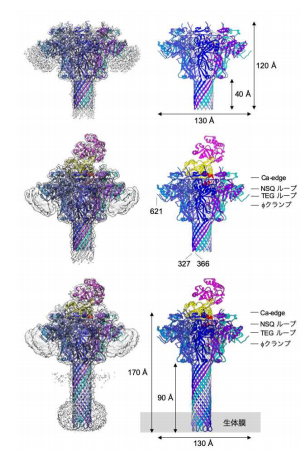
Figure 2
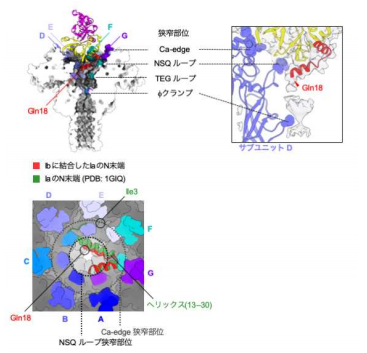
Figure 3
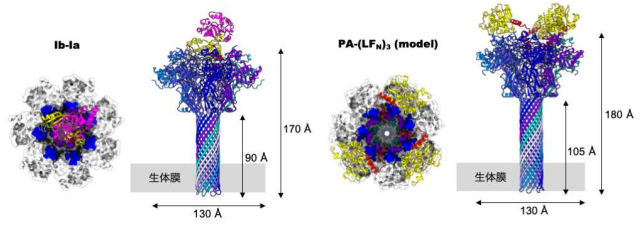
Figure 4
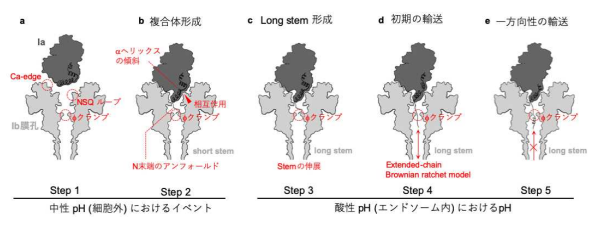
Figure 5
The article, “Cryo-EM structures reveal translocational unfolding in the clostridial binary iota toxin complex” was published in Nature Structural & Molecular Biology at DOI: https://doi.org/10.1038/s41594-020-0388-6
