Light-up wheels: Unique organic light-emitting molecular emitters
Researchers at Osaka University design and synthesize highly efficient carbon-based 3rd-generation OLED molecular emitters by linking the donor and acceptor modules into a ring
A team including researchers from Osaka University has produced a new molecular emitter for organic light-emitting diodes (OLEDs). Using rational chemical design with U-shaped synthetic building blocks, the scientists were able to arrange the electron donors and acceptors into a large ring called a “macrocycle.” The wheel-shaped molecule could potentially be used not only in OLEDs but also in tiny, energy-efficient chemical sensors in the future.
Many modern televisions and smartphones use OLEDs to display pictures and videos. These devices can efficiently convert electricity into light because they are made from carbon-based molecules containing alternating single and double chemical bonds, an arrangement called p-conjugation. This configuration allows electrons to become highly mobile because they are effectively “delocalized” over large regions of the molecules, which tend to be long linear chains. When a molecule is electronically excited by external energy and then relaxes to the original state, the excess energy can be converted directly into light. By adding the right chemical functional groups to the molecule, a whole range of properties, such as emission colors and energy conversion efficiencies, can be fine-tuned.
Now, a research team led by Professor Youhei Takeda at Osaka University has designed and synthesized an efficient macrocyclic OLED emitter in which donor and acceptor regions alternate in a permanently bonded ring structure. They found that OLED devices fabricated with the new macrocyclic emitter show much better efficiencies compared with linear molecular emitters (which act like open forms of the macrocycles), due to the fact that the macrocycles can more efficiently harvest ambient heat energy in a process called “thermally activated delayed fluorescence.”
“Linear p-conjugated oligomers and polymers already play crucial roles in materials science, but we found ring-shaped macrocycles to be even better for many applications,” says first author Saika Izumi. The team was able to create two different conformations, “saddle” and “helical”, with different packing arrangements and emission colors. The nanoscale cavities inside the rings can be designed to interact with target molecules to create efficient and selective chemical sensors.
“Macrocycles can be arranged into highly-ordered 2D- and 3D-molecular assemblies that are much more difficult to achieve with linear analogs,” explains senior author Youhei Takeda.
Possible future applications include the detection of chemical substances, such as water molecules or gases, based on the modulation of light emitted when the target substance is present inside the cavity.
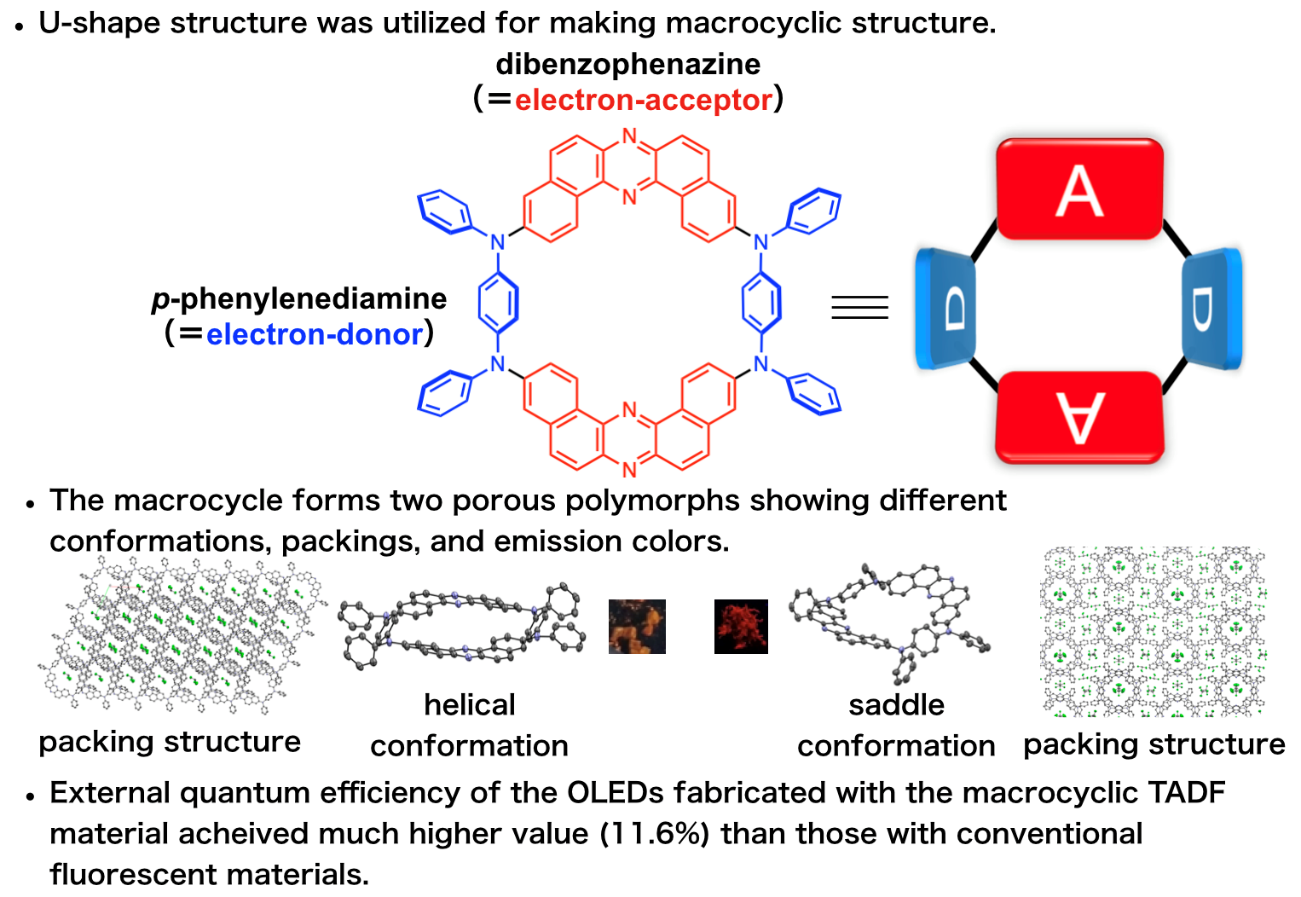
Fig. 1 Schematic explanation for the developed TADF-active macrocyclic material

Fig. 2 Structures of TADF molecular materials
The article, “Thermally activated delayed fluorescent donor–acceptor–donor–acceptor π-conjugated macrocycle for organic light-emitting diodes,” was published in the Journal of the American Chemical Society at DOI: https://doi.org/10.1021/jacs.9b11578 .
Related links
