Spin transfer in organic molecules: First step toward quantum operation using molecules
A group of researchers from The University of Tokyo, Tohoku University, Osaka University, the Institute for Molecular Science, and the Japan Synchrotron Radiation Research Institute observed spin Hall magnetoresistance in a system comprising platinum (Pt) and Fe(II)-phthalocyanine (FePc) molecules, confirming spin transfer from metal (Pt) to molecule (FePc). Phthalocyanine, an organic compound used in paints and dyes, has magnetic properties.
Spintronic technologies use the magnetic moment of an electron caused by its spin. They are used for non-volatile memory and logic circuits, both of which do not require a connection to a power source to retain information. Since spintronic devices consume little electricity and generate practically no heat, they are anticipated as key to lower energy consumption.
To use spin for quantum operation, it is necessary to maintain a spin coherence time of at least 1 microsecond. Spin in molecules can maintain a relatively long coherence time; however, there were no methods for the precise control and read-out of electron spins in molecules, which is necessary for using spin for integrated circuits.
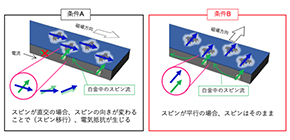
The injection of a spin-polarized current into a ferromagnetic layer causes spin angular momentum transfer between conduction electron spin and localized spin. This angular momentum transfer exerts a spin-torque on the local magnetization, changing its direction. The injection of spin-polarized electric currents into a ferromagnetic/nonmagnetic hybrid structure causes spin Hall magnetoresistance (SMR); that is, resistivity changes by an applied magnetic field due to the spin transfer torque (STT) effect.
A spin current is a flow of spin angular momentum. Spin injection into a ferromagnet transfers spin-angular momentum of spin current to magnetization, enabling control of magnetization using a spin current. This phenomenon caused by STT is used for producing Magnetoresistive Random Access Memory (MRAM).
In experiments, this group detected SMR in a Pt (6 nm)/FePc device, in which the Pt surface was covered by FePc molecules, and then confirmed, using the soft X-ray beamline BL25SU of SPring-8 and theoretical calculations, that the spin state in FePc was maintained in film structures on the FePc device as well.
They measured changes in magnetoresistance and found resistivity changes: magnetoresistance of Pt/FePc under in-plane (xy) magnetic field increased and the magnetoresistance in the y-direction decreased under a perpendicular magnetic field. This phenomenon indicates that SMR occurred.
From these findings, they concluded that spin transfer from metal (Pt) to molecule (FePc) took place. Because the SMR effect arose from spin-transfer processes across the interface between Pt and FePc, spin transfer from Pt to FePC means that the electric control of electron spin in molecules is possible.
This study shows that spin transfer takes place not only in magnetic materials but also in organic molecules. This means that a magnetic moment in FePc can be electrically controlled using STT. Since organic molecules have a sufficiently long coherence retention time, spin transfer in molecules may be used as a bit initialization technology for quantum computers.
Figure 1
Figure 2
The article, “Detection of spin-transfer from metal to molecule by magnetoresistance measurement,” was published in Nano Letters at DOI: http://dx.doi.org/10.1021/acs.nanolett.9b03110 .
Related Links
