Mechanism of CO2 hydrogenation on Cu catalytic surfaces clarified
Molecular vibration drives chemical reactions
A group of researchers from the University of Tsukuba and Osaka University have clarified that CO 2 hydrogenation on Cu catalytic surfaces was a molecular vibration-driven reaction of CO 2 .
In intermolecular chemical reactions in the gas phase, so-called ‘state-to-state chemistry,’ or precise control of chemical reactions by supplying energy to specific modes of the molecular reactant, has been studied. This concept is very important in reducing energy costs of heterogeneous catalysts for use in chemical synthesis in terms of using energy only where necessary.
These types of chemical reactions can be realized by making the reactions between gaseous molecules and catalytic surfaces a thermally non-equilibrated state. Such reactions and their dynamics have been studied using supersonic molecular beam techniques with systematically controlled translational, vibrational, and rotational energies of molecules in the gas phase.
As shown in Fig. 1, the thermal non-equilibrium state gas–surface reactions can be categorized into four types: translational energy-driven dissociation (Fig. 1a), translational energy-driven association (Fig. 1b), vibrational energy-driven dissociation (Fig. 1c), and vibrational energy-driven association (Fig. 1d).
In association, in which vibrational and translational energies of gas molecules affect dissociation of molecules on the solid surface and the gas-phase reactant collides directly with a surface-adsorbed species, the translational energy of gaseous molecules can affect reactivity. However, a bond-formation reaction driven by the vibrational energy of gaseous molecules shown in Fig. 1d (Eley–Rideal (E–R)-type reactions) has not been reported.
The E–R-type mechanism has been suggested for the formation of formate on a Cu surface. Density functional theory (DFT) calculations show that the formation of formate on a Cu surface can be explained by the E-R-type mechanism, in which CO 2 directly attacks a hydrogen adatom on the Cu surface, suggesting the possibility that the formation is driven by O–C–O bending vibration as well. Thus, the researchers tried to verify the E-R mechanism to reveal the dynamics of activation of CO 2 by examining the effects of translational/vibrational energies of CO 2 on CO 2 hydrogenation.
Using supersonic molecular beam techniques that can systematically control translational, vibrational, and rotational energies of gaseous molecules, they examined the hydrogenation reaction of CO 2 on Cu surfaces. As a result, they found that the reaction rate drastically increased by using vibrational energy of CO 2 and that the reaction rate was independent of the surface temperature. This shows that the bond-formation reaction is driven by the vibrational energy of reactant molecules.
The results of this study will deepen the understanding of mechanisms behind various CO 2 -related surface reactions and kinetics. Their research results have opened the door to methanol synthesis from hydrogenation of CO 2 using the energy-saving process to which ‘state-to-state chemistry’ is applied.
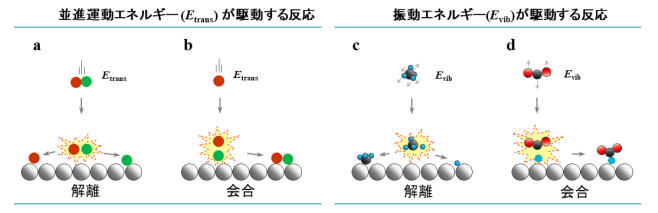
Figure 1

Figure 2

Figure 3

Figure 4

Figure 5
The article, "Vibration-driven reaction of CO 2 on Cu surfaces via Eley–Rideal-type mechanism" was published in Nature Chemistry at DOI: https://doi.org/ 10.1038/s41557-019-0282-1 .
Related links
