The secret adaptive power of photosynthetic complex I is finally revealed
An international team including researchers from Osaka University has explored the structure and function of photosynthetic complex I
Photosynthesis is so well-known that it is likely the first biological process most people learn about in school. Yet, despite this familiarity, until recently finer details of the photosynthetic pathway remained a mystery. Researchers from the Max Planck Institute for Biochemistry, Ruhr-Universität Bochum and Osaka University together with their collaboration partners have used cryo-electron microscopy, X-ray crystallography and NMR spectroscopy to reveal the structural adaptation of photosynthetic complex I, a membrane protein complex involved in electron transport. Their work, published in Science, finally addresses one of the few remaining questions for photosynthetic electron transport pathways.
Complex I plays two different roles in plant cells. Mitochondrial complex I, which participates in electron transport in the energy conversion powerhouses of cells, is well-characterized in terms of structure and function. However, photosynthetic complex I, which is present in chloroplasts and contributes to the transfer of electrons in photosynthesis, has not been widely studied.
“The resolution provided by cryo-electron microscopy shows that photosynthetic complex I has a notably different structure to mitochondrial complex I, its respiratory counterpart,” study author Hideaki Tanaka explains. “Revealing these differences in structure has helped us to understand the function of photosynthetic complex I on a molecular level and its role in electron transport.”
Photosynthesis requires cyclic electron flow, where electrons are reinjected into the system rather than be used for Carbon fixation to balance the electron distribution in the cell. By replicating this process in experiments using detergent-solubilized photosynthetic complex I the researchers have been able to show that there is a direct and efficient interaction between photosynthetic complex I and the electron carrier ferredoxin.
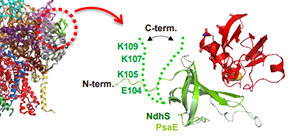
By performing additional X-ray crystallographic and NMR spectroscopic experiments, the team was able to identify a certain part of the photosynthetic complex I structure that is particularly flexible, making it suitable for interaction with ferredoxin. The protein subunit arrangement at this point makes conditions conducive to both the binding and subsequent electron transfer required for an efficient interaction.
“Understanding the structural interaction of protein complexes and their roles in particular pathways is a necessary starting point for developing synthetic processes in the cell,” says corresponding author Genji Kurisu. “Our findings fill in some longstanding gaps that will allow areas such as the artificial optimization of photosynthesis and the enhancement of photosynthetic function to be furthered.”

Fig.1
Photosynthetic electron transport chain: The electrons originated from water splitting are re-injected into the thylakoid membrane by the electron carrier ferredoxin (Fd) through the photosynthetic complex I (NDH1) whose structure was not known.

Fig.2
Cryo-EM structure of NDH1 and the structural model of the complex formation with ferredoxin (Fd): Left panel shows the cryo-EM structure of NDH1. Right panel shows the possible interaction mode modeled by X-ray structure of NdhS subunit and NMR spectroscopic analysis of the interaction between NdhS and Fd.
The article, “Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer” was published in Science at DOI: https://doi.org/10.1126/science.aau3613 .
Related links
