Calredoxin, a novel protein for promoting efficient photosynthesis
A key player behind the reduction of oxidative stress in photosynthetic organisms
Outline
A group of researchers reported on the structure and function of a novel protein named “Calredoxin.” Calredoxin binds calcium and catalyzes in dependence of its binding, redox reactions, particularly driving the detoxification of harmful oxygen species. The researchers are exploring how this protein functions at the crossroad of calcium- and redox-dependent reactions to promote efficient oxygenic photosynthesis.
Background (including information on researchers)
The conversion of solar energy into chemical energy and building material from inorganic compounds by oxygenic photosynthesis supports much of the life on this planet. In plants, these reactions are situated in the chloroplast. The production of oxygen and the assimilation of carbon dioxide into organic matter determines, to a large extent, the composition of our atmosphere.
To cope with varying light intensities, oxygenic photosynthetic organisms have evolved acclimation strategies that are aimed to optimize photosynthetic performance and diminish oxidative stress. Harmful oxygen species, like reactive oxygen species (ROS), are particularly generated via photosynthesis under fluctuating light and high light stress. In the latest issue of Nature Communications , an international team around Prof. Michael Hippler from the Institute of Plant Biology and Biotechnology (WWU Münster, Germany) and Prof. Genji Kurisu from the Institute of Protein Research (Osaka University, Japan) report on the structure and function of a novel protein named “Calredoxin” (Fig.1).
Calredoxin binds calcium and catalyzes in dependence of its binding, redox reactions. Especially, in the detoxification of ROS, redox reactions play a crucial role. Calcium is an essential element in oxygenic photosynthesis and also critically involved in the regulation of high light stress. Calredoxin was discovered in the chloroplast of the green alga Chlamydomonas reinhardtii and is e.g. involved in the detoxification of ROS mediated by Peroxiredoxin under light stress.
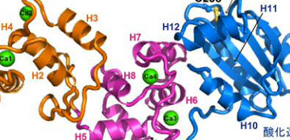
The X-ray structure of Calredoxin with bound calcium ions were successfully solved at 1.6 Å resolution, whose architecture is suitable for intramolecular communication of calcium (Fig.1A, orange, pink) and redox signaling (Fig. 1A, blue) and inter-molecular interaction with Peroxiredoxin (Fig. 1B). Thereby Calredoxin functions at the crossroad of calcium- and redox-dependent reactions to promote efficient oxygenic photosynthesis (Fig.2).
Address to the society
Limitation of the world’s crop productivity is a serious problem. One latent possibility to solve this problem is increasing the efficiency of photosynthesis. A principle limitation of efficient photosynthesis is that organisms absorb more light in full sunlight than they can use effectively, thus plants have evolved a variety of mechanisms for photoacclimiation, including a mechanism described in the current new findings. Although our findings are to the level of basic research, they may help addressing the agricultural crisis to redesign photosynthesis that would be a big scientific challenge for us.
Comments/messages from researcher(s)
Our collaboration between Osaka and Münster is really complementary, because OU does not have agricultural faculties and WWUM does not have a structural biology department.

Figure 1. Structure of Calredoxin (A) and interaction model with Peroxiredoxin (PDB ID: 3VWU) involved in the antioxidant metabolism.
Figure 2. Calredoxin as a signal-responder at the crossroad of Ca 2+ - and redox-signaling
To learn more about this research, please view the full research report entitled “ Calredoxin represents a novel type of calcium-dependent sensor-responder connected to redox regulation in the chloroplast ” at this page of the Nature Communications website.
Related links
