
Environmentally Friendly Photoluminescent Nanoparticles for More Vivid Display Colors
Researchers create new type of light-emitting nanoparticle made of ternary non-toxic semiconductors to help create displays and LED lighting with better colors that are more environmentally friendly
Most current displays do not always accurately represent the world's colors as we perceive them by eye, instead only representing roughly 70% of them. To make better displays with true colors commonly available, researchers have focused their efforts on light-emitting nanoparticles. Such nanoparticles can also be used in medical research to light up and keep track of drugs when developing and testing new medicines in the body. However, the metal these light-emitting nanoparticles are based on, namely cadmium, is highly toxic, which limits its applications in medical research and in consumer products—many countries may soon introduce bans on toxic nanoparticles.
It is therefore vital to create non-toxic versions of these nanoparticles that have similar properties: they must produce very clean colors and must do so in a very energy-efficient way. So far researchers have succeeded in creating non-toxic nanoparticles that emit light in an efficient manner by creating semiconductors with three types of elements in them, for example, silver, indium, and sulfur (in the form of silver indium disulfide (AgInS2)). However, the colors they emit are not pure enough—and many researchers declared that it would be impossible for such nanoparticles to ever emit pure colors.
Now, researchers from Osaka University have proven that it is possible by fabricating semiconductor nanoparticles containing silver indium disulfide and adding a shell around them consisting of a semiconductor material made of two different elements, gallium and sulfur. The team was able to reproducibly create these shell-covered nanoparticles that are both energy efficient and emit vivid, clean colors. The team have recently published their research in the Nature journal NPG Asia Materials .
"We synthesized non-toxic nanoparticles in the normal way: mix all ingredients together and heat them up. The results were not fantastic, but by tweaking the synthesis conditions and modifying the nanoparticle cores and the shells we enclosed them in, we were able to achieve fantastic efficiencies and very pure colors," study coauthor Susumu Kuwabata says.
Enclosing nanoparticles in semiconductor shells in nothing new, but the shells that are currently used have rigidly arranged atoms inside them, whereas the new particles are made of a more chaotic material without such a rigid structure.
" The silver indium disulfide particles emitted purer colors after the coating with gallium sulfide. On top of that, the shell parts in microscopic images were totally amorphous. We think the less rigid nature of the shell material played an important part in that—it was more adaptable and therefore able to take on more energetically favorable conformations," first author Taro Uematsu says.
The team's results demonstrate that it is possible to create cadmium-free, non-toxic nanoparticles with very good color-emitting properties by using amorphous shells around the nanoparticle cores.

Fig. 1. Photoluminescence spectra of iridium complex and conventional cadmium selenide quantum dots
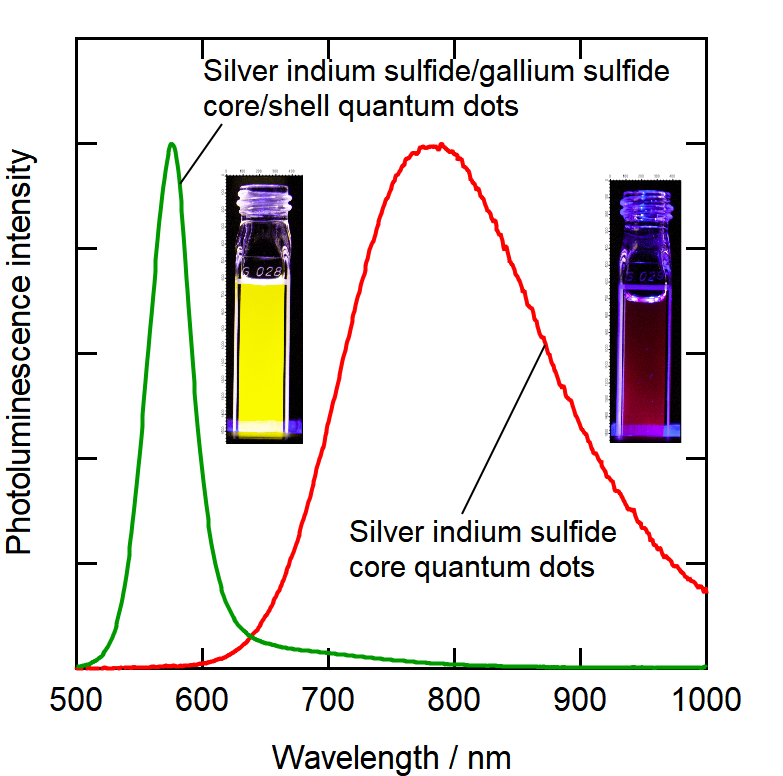
Fig. 2. Silver indium sulfide core quantum dots and those coated with gallium sulfide shell

Fig. 3. Structures of silver indium sulfide/gallium sulfide core/shell quantum dots and pictures of the core/shell quantum dots under room light
The article, “Narrow band-edge photoluminescence from AgInS 2 semiconductor nanoparticles by the formation of amorphous III–VI semiconductor shells” was published in NPG Asia Materials , https://doi.org/10.1038/s41427-018-0067-9 .
Related links
