CD4 T Cells, Xenobiotic Transporters, and Metabolites in Inflammatory Bowel Diseases
International researchers show CD4+ T effector cells express xenobiotic transporter Mdr1 in the presence of bile acids to maintain homeostasis in the small intestine
Our immune system protects our bodies from numerous pathogenic microbes and toxins in the environment. The system comprises innate (non-specific) and adaptive (acquired) immunity. When innate immune cells recognize pathogens, the adaptive immune system is called into action. There are two types of adaptive immune responses: humoral immunity mediated by antibodies produced by B cells, and cell-mediated immunity mediated by T cells, a type of white blood cells.
CD4+ T cells regulate/suppress immune responses through several mechanisms.“naïve” CD4 T cells can differentiate into one of several lineages of effector (Teff) cells, including Th1 and Th17. In disease-free state, Teff cells are tightly regulated by intestinal microbiota, which is the community of microorganisms that normally inhabits the digestive tracts of humans and other animals. However, when there is an accumulation of Th17 and Th1 cells in the intestine, the symbiotic relationship may be disrupted, leading to conditions such as inflammatory bowel diseases (IBDs), a group of debilitating and sometimes leads to life-threatening conditions that affect the colon and small intestine.
While a great deal is known about the mechanisms underlying the symbiotic interactions between Teff cells and intestinal microbiota, far less is known about the interaction of Teff cells with metabolites, which are the intermediates and products of metabolism. This motivated an international team of researchers centered at Japan’s Osaka University to fill the knowledge gap, potentially yielding valuable clues for development of robust therapeutic agents against IBDs.
For their study, the researchers focused on bile acid, a detergent-like metabolite produced by the catabolism of cholesterol catabolism in the liver and deposited through the bile duct into the small intestine in response to food intake. Bile acids have been reported to induce oxidative stress in liver and epithelial cells by perturbing normal cell membrane architecture.
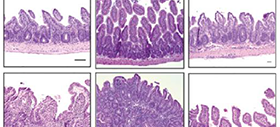
“We observed in mice that Teff cells upregulate the protein Mdr1, in the end portion of the small intestine (ileum) to maintain homeostasis in the presence of bile acids,” says Hisako Kayama, from Osaka University and author of the study recently published in The Journal of Immunology. “Notably, those lacking Mdr1 display mucosal dysfunction and induce Crohn’s disease-like ileitis.”
Mdr1 is an important protein that is extensively distributed and expressed in the intestinal cells. It pumps xenobiotics (such as toxins or drugs) out of the cell, and has essential functions in Teff cells during bile acids exposure, limiting oxidative stress, promoting survival, and suppressing inflammatory cytokine expression. The team noted the inhibition of bile acids reabsorption in the ileum restores mucosal homeostasis in mice transplanted with Mdr1-deficient Teff cells, which underscores the involvement of Teff dysregulation in the pathogenesis of IBD.
“We have also successfully identified a discernable subset of ileal Crohn’s disease patients who display marked MDR1 loss-of-function in the presence of bile acids,” Kayama adds. “As such, future validation studies of larger IBD patient cohorts is important to test whether an Mdr 1 loss-of-function is associated with a unique clinical course of ileal CD. Such knowledge may present opportunities for non-traditional, bile acids-directed therapy.”
Abstract
Intestinal CD4 + T helper (T H ) cells are subject to extensive regulation by microbiota. By contrast, it is not known whether or how T H cells interface with other, host-derived intestinal metabolites. Here we show that bile acids directly regulate mucosal T H cell function in the distal small intestine (i.e., ileum) via the xenobiotic transporter, Mdr1. Using both Mdr1-dependent dye efflux and a novel CRISPR-generated Mdr1 reporter mouse, we show that wild type RORγt + IL-17A + (Th17) and RORγt-IFNγ + (Th1) cells upregulate Mdr1 expression upon migration into the ileum. By contrast, germline ablation or shRNAmir-mediated knockdown of Mdr1 in Th17 and Th1 cells results in local dysfunction in the ileum, and these cells transfer Crohn’s disease-like ileitis in Rag1 −/− hosts. Mdr1 enforces Th17 and Th1 cell survival and limits pro-inflammatory cytokine (TNFα, IFNγ) expression in the presence of conjugated bile acids (CBAs), which are actively reabsorbed through the ileal mucosa as a function of enterohepatic bile acid circulation. Accordingly, genetic or pharmacologic blockade of ileal CBA reabsorption restores Mdr1-deficient Th17 and Th1 cell homeostasis in ilea of transferred Rag1 −/− hosts and rescues ileitis. In addition, MDR1 loss-of-function is evident in both ileitis-prone (SAMP1/YitFc) mice, and a subset of ileal Crohn’s disease patients. These data indicate that coordinated, local and druggable interactions between mucosal T H cells and mucosa-associated bile acids in the ileum contribute to intestinal immune homeostasis.
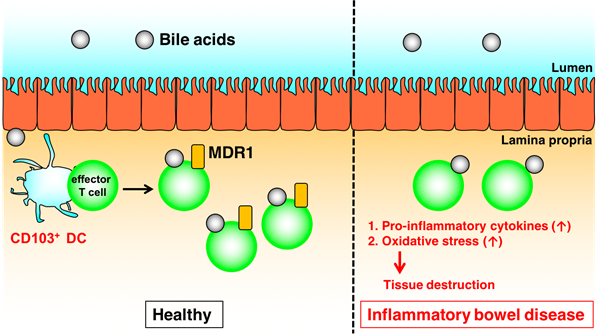
Fig.1. Mdr1 induced by CD103+ DCs mediates maintenance of intestinal T cell homeostasis in the presence of bile acids (credit: Osaka University)
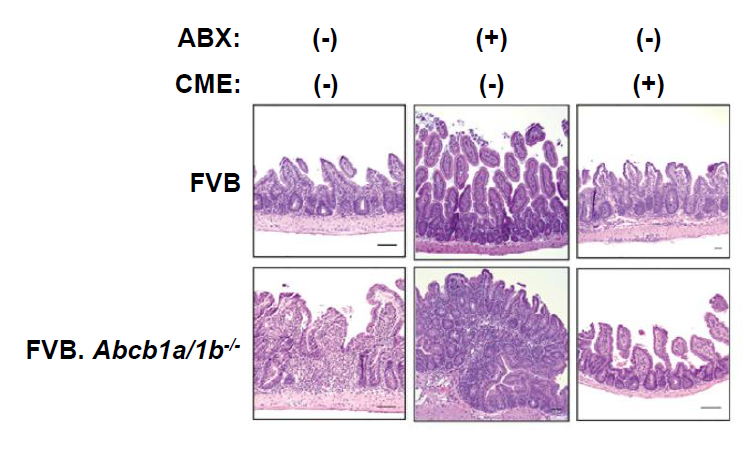
Fig.2. Bile Acid Sequestration Restores Mucosal Homeostasis of Mdr1-Deficient T Cells
(credit: Osaka University and The Scripps Research Institute)
To learn more about this research, please view the full research report entitled " The xenobiotic transporter Mdr1 permits T cell adaptation to bile acid reabsorption in the ileum " at this page of Immunity .
Related links
