Japanese researchers find why Alzheimer’s drugs work in the lab but not in patients
A tremendous amount of Amyloid-β peptide (Aβ) (a peptide of ~40 amino acids) accumulates in the brain of Alzheimer’s disease patients. γ-Secretase inhibitors were designed to inhibit the enzymatic activity that produces Aβ. By reducing Aβ production, γ-secretase inhibitors were considered able to treat Alzheimer’s disease (Aβ hypothesis). In fact, nearly 50 clinical trials have been conducted using potential γ-secretase inhibitors for Alzheimer’s disease or several types of cancer. However, all of these trials have failed, except for two studies which are currently ongoing.
Osaka University scientists found that some potential γ-secretase inhibitors such as semagacestat, which have been used in large clinical trials that ended in failure, do not function as true inhibitors as originally expected, but rather cause accumulation of toxic intraneuronal Aβ. They proved this by introducing an original method to measure direct intracellular products of γ-secretase. They commented that the application of their evaluation method may help develop truly effective drugs for Alzheimer’s disease (AD). The study can be seen in Cell Reports and provides an explanation for why the clinical trials for Alzheimer’s disease drugs have failed and gives new light on the discord between preclinical and clinical findings.
“Aβ accumulates in the brain at the very early stages of Alzheimer’s disease,” explains Osaka University Associate Professor Masayasu Okochi, an expert on the disease who managed the project. “Aβ generation is based on the activity of presenilin/γ-secretase which mediates the cellular production of Aβ.”
Of the promising sets of drugs for Alzheimer’s disease were γ-secretase inhibitors like semagacestat. However, a clinical trial that began almost 10 years ago was terminated early because not only was semagacestat found to fail, patient groups that received the drug showed exasperated symptoms compared to the placebo group. This finding has put great doubt into the Aβ hypothesis.
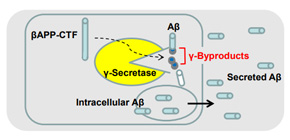
To understand this surprising finding, Okochi considered whether semagacestat is indeed a β-secretase inhibitor. In this study, he and colleagues revealed that semagacestat does not really inhibit the target function, i.e., the cleavage performed by γ-secretase (γ-cleavage) (Figure GA). What enabled them to reach this unexpected finding is an original method that this team established, which can measure direct products of γ-secretase (peptides of 3 to 5 small amino-acid residues which were named γ-byproducts) (Figure 2A and 3E).
Surprisingly, non-transition state analogue γ-secretase inhibitors including semagacestat did not decrease, but rather increased the levels of γ-byproducts (Figure 3D). This finding shattered the belief that these compounds truly inhibit the proteolytic function of γ-secretase and made the researchers “look” inside neurons for further assessment. As predicted from the increased level of γ-byproducts, an accumulation of Aβ was found inside neurons derived from human iPS cells and various types of cultured cells (Figure 3B and 3C),), although semagacestat did in fact decrease secreted Aβ, as has been previously reported (Figure 3A).
These results suggested to Okochi that semagacestat is not in fact a γ-secretase inhibitor, which is why he uses the term “pseudo γ-secretase inhibitor” in the study. Clinical tests of semagacestat tended to judge the drug based on Aβ secretion but not γ-byproducts, which could explain why pseudo γ-secretase inhibitors have been repeatedly mislabeled.
“We found the type of assay gives different results. In our assay, we found γ-byproducts in the cell membrane. Semagacestat may prevent release of γ-byproducts from the membrane but not the generation of γ-byproducts,” he said.
Ironically, notes Okochi, considering his findings, he argues the failed clinical trials affirm the Aβ hypothesis.
“I believe normalization of production and secretion of Aβ by sharpening γ-secretase is the right approach to treating Alzheimer’s disease. Our tests suggest that molecularly targeted therapy should be thoroughly checked from all angles before its application to clinical studies. The new function of γ-secretase suggested in this study needs further analysis, which will contribute to the development of truly effective drugs for Alzheimer’s disease and several types of cancer,” he said.

Figures,
Figure GA. Schematic summary of this study
Figure 2A. Peptides of 3 to 5 small amino-acid residues which we named as γ-byproducts.
Figure 3D. Semagacestat did not decrease, but rather increased the levels of γ-byproducts
Figure 3E. The increasing effect of γ-byproducts by semagacestat is cancelled by knockdown of presenilin genes.
Figure 3A. Semagacestat did in fact decrease secreted Aβ, as has been previously reported.
Figure 3B and 3C. An accumulation of Aβ was found inside cells.
(©2017 Tagami et al. Cell Reports 21, Issue1, 259-273. doi: 10.1016/j.celrep.2017.09.032)
To learn more about this research, please view the full research report entitled " Semagacestat is a Pseudo-Inhibitor of γ-Secretase " at this page of Cell Reports .
Related links
