Discovery of a lipid that senses tissue damage and induces immune responses
Will lead to the development of cures for Gaucher's disease and Parkinson’s disease
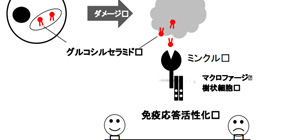
A group of researchers led by Professor YAMASAKI Sho at the Medical Institute of Bioregulation, Kyushu University and Research Institute for Microbial Diseases, Osaka University, discovered that β-glucosylceramide (β-GlcCer) lipid, bound to Macrophage inducible C-type lectin (Mincle), an innate immune receptor, and activated immune systems.
When cells are damaged, immune systems are activated in order to remove damaged cells; however, how this happens was unknown. This group examined lipid released from damaged cells, finding that β-GlcCer bound to Mincle.
Normally, a constant level of β-GlcCer is maintained by a degrading enzyme; however, when this enzyme is defective, β-GlcCer accumulates, developing Gaucher's disease and Parkinson’s disease. It was also known that these diseases were associated with chronic inflammation; however, why accumulation of β-GlcCer led to inflammation was unknown.
This group elucidated its mechanism and showed the possibility that inhibition of Mincle would lead to treatment of these diseases. The researchers plan to develop treatment methods by examining effects of treatment when Mincle is inhibited in animal models of Gaucher’s disease and Parkinson’s disease.
Abstract
Sensing and reacting to tissue damage is a fundamental function of immune systems. Macrophage inducible C-type lectin (Mincle)is an activating C-type lectin receptor that senses damaged cells. Notably, Mincle also recognizes glycolipid ligands on pathogens. To elucidate endogenous glycolipids ligands derived from damaged cells, we fractionated supernatants from damaged cells and identified a lipophilic component that activates reporter cells expressing Mincle. Mass spectrometry and NMR spectroscopy identified the component structure as β-glucosylceramide (GlcCer), which is a ubiquitous intracellular metabolite. Synthetic β-GlcCer activated myeloid cells and induced production of inflammatory cytokines; this production was abrogated in Mincle-deficient cells. Sterile inflammation induced by excessive cell death in the thymus was exacerbated by hematopoietic-specific deletion of degrading enzyme of β-GlcCer (β-glucosylceramidase, GBA1). However, this enhanced inflammation was ameliorated in a Mincle-deficient background. GBA1-deficient dendritic cells (DCs) in which β-GlcCer accumulates triggered antigen-specific T-cell responses more efficiently than WT DCs, whereas these responses were compromised in DCs from GBA1 × Mincle double-deficient mice. These results suggest that β-GlcCer is an endogenous ligand for Mincle and possesses immunostimulatory activity.
To learn more about this research, please view the full research report entitled " Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity " at this page of the PNSA website.
Related link
