Discovery of materials to selectively intercalate heavy metals in solid state
A step forward to the development of new methods for recovering metals from electronic devices
A group of researchers led by former graduate student EKO Masataka, former researcher YAJIMA Takeshi , former researcher Yaoqing Zhang , and Professor KAGEYAMA Hiroshi at the Graduate School of Engineering, Kyoto University, in joint research with Professor OGUCHI Tamio at The Institute of Scientific and Industrial Research, Osaka University, discovered that titanium layered compounds could selectively intercalate heavy metals such as cadmium at low temperatures.
Metal recovery from waste electronic devices is quite important in terms of securing energy resources and preventing environmental contamination. A reaction in which metal molecules are inserted into compounds (intercalation reaction) has been observed in various layered compounds such as graphite and clays; however, selective intercalation of specific metals was difficult to achieve.
This group discovered that titanium layered compounds selectively intercalated cadmium, copper, and zinc. This group also found that, unlike conventional materials, intercalation reactions proceeded in titanium layered compounds by just slightly increasing the temperature.
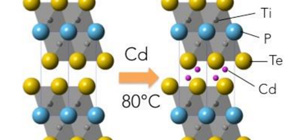
Generally, layers in layered compounds are connected by weak interactions, so not only various metal elements, but also large organic molecules are intercalated. So it was strange that selective metal intercalation was observed in phosphide telluride Ti 2 PTe 2 , a layered compound. As a result of theoretical calculation, it was found that a local structure in which there is titanium bound with different anions (phosphorus and tellurium) gave a unique electronic structure, enabling selective metal intercalation. In layered compound titanium telluride (TiTe 2 ) in which titanium is bound with only tellurium, such selectivity is not observed.
Another interesting point is that metal intercalation of Ti 2 PTe 2 proceeded in a solid state at low temperatures. Although ordinary solid reactions start at 1,000°C, in Ti2PTe2, reactions started at low temperatures -- 80°C for cadmium and 100°C for zinc. Inorganic matter including oxides are synthesized at high temperatures of around 1,000°C in order to promote diffusion of metal atoms. There are few examples of diffusion of heavy metals at low temperatures as shown in Ti 2 PTe 2 , so this group’s achievement will lead to new developments in low temperature operation and efficiency of solid fuel cells.
This study presented a new type of methodology for collecting metals. Cadmium is essential for electronic devices. Its use is increasing year by year in spite of its toxicity. Technology for taking out necessary or unnecessary metals from materials will become more important than ever. It is hoped that research and development of materials capable of selectively intercalating toxic metals and rare metals will be promoted based on the findings in this study.
Abstract
Layered materials embrace rich intercalation reactions to accommodate high concentrations of foreign species within their structures, and find many applications spanning from energy storage, ion exchange to secondary batteries. Light alkali metals are generally most easily intercalated due to their light mass, high charge/volume ratio and in many cases strong reducing properties. An evolving area of materials chemistry, however, is to capture metals selectively, which is of technological and environmental significance but rather unexplored. Here we show that the layered telluride T 2 PTe 2 (T=Ti, Zr) displays exclusive insertion of transition metals (for example, Cd, Zn) as opposed to alkali cations, with tetrahedral coordination preference to tellurium. Interestingly, the intercalation reactions proceed in solid state and at surprisingly low temperatures (for example, 80 °C for cadmium in Ti 2 PTe 2 ). The current method of controlling selectivity provides opportunities in the search for new materials for various applications that used to be possible only in a liquid.
To learn more about this research, please view the full research report entitled “ Selective and Low Temperature Transition Metal Intercalation in Layered Tellurides ” at this page of the Nature Communications website.
Related link
