Identification of autophagy-dependent secretion machinery
A novel potential for therapeutic approach
Outline
A group of researchers identified a molecular machinery by which autophagy *1 mediates secretion. These results underscore an important role of autophagy other than degradation, and will bring us to future translational research of medicine.
Background
Autophagy has long been considered as a physiological process solely for degradation, but its secretory role has recently emerged. A group of researchers, including Tomonori Kimura, a researcher at the Department of Nephrology, Osaka University (the research was conducted at the University of New Mexico, USA), identified the molecular machinery by which autophagy mediates secretion of the inflammatory cytokine *2 , interleukin-1 beta, in corporation with SNARE proteins *3 .
Address to the society
In addition to interleukin-1 beta, leaderless proteins such as ferritin, whose secretory system has not been identified, are also found to be secreted based on the same autophagy machinery. Therefore, the newly-identified autophagy-dependent secretory system facilitates the secretion of leaderless proteins *4 , and plays fundamental roles in this secretion.
Comments/messages from researcher(s)
Autophagy is related with a diverse spectrum of diseases and has long been anticipated as a therapeutic option from the point of view of degradation. Our findings will open another dimension for therapeutic approaches through the secretory role of autophagy.
Abstract
Autophagy is a process delivering cytoplasmic components to lysosomes for degradation. Autophagy may, however, play a role in unconventional secretion of leaderless cytosolic proteins. How secretory autophagy diverges from degradative autophagy remains unclear. Here we show that in response to lysosomal damage, the prototypical cytosolic secretory autophagy cargo IL ‐1β is recognized by specialized secretory autophagy cargo receptor TRIM 16 and that this receptor interacts with the R‐ SNARE Sec22b to recruit cargo to the LC 3‐ II + sequestration membranes. Cargo secretion is unaffected by downregulation of syntaxin 17, a SNARE promoting autophagosome–lysosome fusion and cargo degradation. Instead, Sec22b in combination with plasma membrane syntaxin 3 and syntaxin 4 as well as SNAP ‐23 and SNAP ‐29 completes cargo secretion. Thus, secretory autophagy utilizes a specialized cytosolic cargo receptor and a dedicated SNARE system. Other unconventionally secreted cargo, such as ferritin, is secreted via the same pathway.

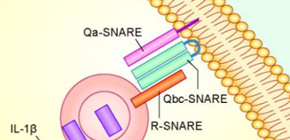
Figure. IL-1β within autophagosome is secreted from cytoplasm in corporation with SNARE proteins.
*1 Autophagy
Autophagy is a self-degradative process for cellular homeostasis. Autophagosome sequesters the degradative targets, whose process is followed by fusion with lysosomes for degradation of its contents. Recently, autophagy is also reported to play a role in secretion.
*2 Inflammatory cytokine
A subset of cytokines (proteins secreted from cells to mediate information to other cells) which promotes inflammation. Interleukin-1 beta is a major inflammatory cytokine which is activated and released upon infection.
*3 SNARE proteins
SNARE proteins mediates vesicular fusion, such as fusion between cellular plasma membrane and cytoplasmic vesicles. This fusion is mediated by a set of SNARE proteins, generally consisting of Qa-, Qb-, Qc-, and R-SNARE proteins.
*4 Leaderless protein
Signal peptides are short peptides in proteins through which proteins are bound for specific cellular distribution or secretion. A number of proteins do not have signal peptides (called leaderless proteins), and their secretory pathway have not been elucidated thus far.
To learn more about this research, please view the full research report entitled “ Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy ” at this page of the EMBO Journal website.
Related links
