Dibenzoazepine Defender: Drug Found to be Effective Against Resistant Hepatitis C
Osaka University researchers identify class of chemicals that can combat resistant strains of the hepatitis C virus, as well as parasites that cause malaria and toxoplasmosis
Hepatitis C is caused by a highly infectious virus affecting millions across the globe and can lead to a variety of liver ailments. While the hepatitis C virus (HCV) can sometimes be fought off and cleared by the immune system during the first few months of acute infection, up to 80% of those with HCV develop a chronic infection. This can lead to serious liver illnesses, including inflammation, cirrhosis, and hepatocellular carcinoma—the third leading cause of cancer death worldwide.
While highly effective treatments for HCV have become available in recent years, drug-resistant viral strains can still lead to treatment failure for a sizable proportion of patients. Now, in a recent study published in PNAS Plus , a compound has been reported that may eventually prove effective against drug-resistant HCV.
A team of researchers centered at Osaka University infected human liver cells with HCV, then treated the infected cells with different drugs to see which might prevent the virus from spreading. One compound, innocuously named YO-01027, stood out above the rest.
“For HCV to propagate in a host cell, the proteins that make up the virus particle need to be cleaved into their mature form,” lead author Junki Hirano explains. “We tested several compounds we thought may inhibit this cleavage process, and found that YO-01027 prevents a key HCV protein from undergoing cleavage and maturation. We correspondingly found the drug is very effective at suppressing HCV infection.”
Importantly, resistant strains of HCV did not emerge over time when the infected cells were treated with YO-01027. This may owe to the unique way the compound prevents the virus from maturing.
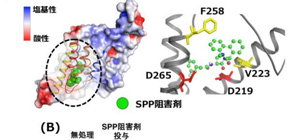
Patients with HCV are currently given direct-acting antivirals, which (as their name suggests) directly target and disrupt HCV proteins themselves. The drug tested in this study, however, inhibits one of the host cell’s proteins—signal peptide peptidase (SPP)—that HCV hijacks during an infection.
“Direct-acting antivirals have made tremendous progress in treating HCV,” corresponding author Yoshiharu Matsuura explains. “The difficulty is that HCV shows quite high genetic diversity, even within a single patient. Antivirals produce a strong selective pressure that can cause HCV strains with resistant forms of the target protein to spread. By inhibiting the host’s own SPP protein, we can largely bypass this selection problem.”
Through a combination of computer simulations and in vitro tests, the researchers identified the chemical signature of YO-01027 responsible for its effectiveness, a structure called dibenzoazepine. With this and other molecular details in hand, the researchers may now be able to modify YO-01027 and other dibenzoazepine-containing drugs to develop novel therapies for drug-resistant HCV—and, serendipitously, to potentially develop therapies against a variety of other diseases.
“Now that we know some of the key structural features that make YO-01027 effective at inhibiting SPP, we can start the chemical fine tuning,” Matsuura adds. “Ultimately, the goal is to make highly selective drugs to combat pathogens that need SPP to survive and spread. This includes not only viruses like HCV, but also parasites such as Plasmodium falciparum and Toxoplasma gondii that are responsible for malaria and toxoplasmosis. The possible applications are very exciting.”

Figure: (Upper panel) Val223 and Phe258 of SPP interacts with the inhibitors for SPP and γ-secretase.
(Lower panel) The inhibitor prevents proliferation of protozoa in toxoplasma infected mice. The inhibitor also ameliorates liver steatosis induced by HCV core protein. (Credit: Osaka University)
To learn more about this research, please view the full research report entitled " Characterization of SPP inhibitors suppressing propagation of HCV and protozoa " at this page of Proceeding of the National Academy of Sciences of the United States of America .
Related links
