Elucidation of mechanism by which Regulatory T-cells control vaccination response
CTLA-4 controls antibody production
Professor Shimon SAKAGUCHI of Osaka University IFReC has found that Regulatory T-cells (Tregs) use the immunoregulatory molecule CTLA-4 to suppress T-follicular helper cells, a T-cell subset that is critical to antibody production. This finding is important to help us control antibody production to develop better vaccines and new treatments for antibody driven autoimmunity.
Introduction
Following infection or vaccination the immune system must produce antibodies that attack pathogens such as viruses and bacteria in order to protect the host. However overactivation of the immune system may lead to the production of antibodies that attack our own bodies and cause autoimmune diseases such as systemic lupus erythematosus (SLE) and Rheumatoid arthritis (RA). Tregs are a specialized set of T-cells that suppress the activation of other cells and loss of Treg function that leads to fatal autoimmunity. Thus Tregs must walk a tightrope in which they allow antibody production to pathogens and following vaccination but prevent the production of autoimmune antibodies targeting the host. However the role of Tregs in the control of antibody producing B-cells was only partially understood. We set out to determine the role of Tregs in control of antibody production following vaccination and the mechanism by which they achieve their effect.
The key points are :
Depletion of Treg cells, and their subset T-follicular regs, leads to enhanced numbers of T-follicular helper cells (Tfh), a T-cell subtype that specializes in delivering help to B-cells and enhances their conversion to antibody producing plasma B-cells. However importantly, only short term depletion of Tregs enhanced antigen specific cells while longer term depletion of Tregs increased autoimmune antibody production, demonstrating the complex role of Tregs in antibody production.
Depletion of Tregs also enhances memory B-cell production and antibody production following later vaccination without further Treg depletion suggesting that a single depletion of Tregs during primary vaccination will lead to stability enhanced immunity for long periods of time.
The mechanism by which Tregs control Tfh numbers is by depletion of stimulatory molecules from the surface of antigen presenting cells (including B-cells) via the co-inhibitory molecule CTLA-4. As a result blocking of CTLA-4 enhanced antibody responses to vaccination. While mice with Treg targeted depletion of of CTLA-4 develop spontaneous formation of large numbers of Tfh and germinal center B-cells, which are the source of plasma and memory B-cells.
These results are important as they will allow us to develop new ways to either decrease Treg function to enhance vaccine responses or boost Treg function to prevent antibody driven autoimmunity.
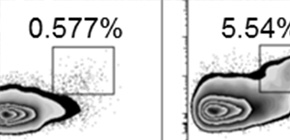
Figure 1 A) Tfh cell (BCL6+CXCR5+) formation in wildtype (WT), Treg depleted (DEREG) or Treg specific CTLA4 deficient mice. B) Enhanced B-cell germinal center, plasma cell and memory B-cell formation in Treg depleted DEREG mice.
To learn more about this research, please view the full research report entitled " Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4 " at this page of the Immunity website.
Related Links
