Cellular ability to repair damaged DNA successfully visualized
This group’s method avoids UV-related reactions such as lipid oxidation
A group of researchers developed a probe for detecting nucleotide excision repair (NER), a mechanism that removes DNA damage induced by ultraviolet light (UV), thereby succeeding in visualizing cellular NER.
• IWAI Shigenori , Professor, KURAOKA Isao , Associate Professor, Graduate School of Engineering Science, Osaka University
• NISHIGORI Chikako , Professor, Graduate School of Medicine, Kobe University
• SUGASAWA Kaoru , Professor, Biosignal Research Center, Kobe University
Patients with xeroderma pigmentosum (XP) have genetic defects in NER and, therefore, have a great risk of developing skin cancer. Therefore, early diagnosis is important. Conventional tests measure unscheduled DNA synthesis by irradiating (with UV light) cultivated skin cells obtained from the subject. That is, through the use of radiation, the unscheduled DNA synthesis assay measures a cell's ability to perform NER.
This group has developed a probe for detecting NER by observing fluorescence emission, thus succeeding in visualizing cellular NER. This probe containing a (6-4) photoproduct, a fluorophore (fuorescein), and a quencher (dabsyl). This method works on the principle that when a damaged strand is incised by NER, it is broken down by nucleolytic enzyme nuclease in cells and fluorophores separate from the quencher, yielding fluorescence.
The probe developed by this group can be inserted using commercial transfection reagent and detect NER; therefore, it will possibly simplify the diagnosis of XP. Furthermore, if leukocytes can be used, it will become possible to investigate without incising the skin, thereby reducing the burden on the subject. Moreover, as conventional tests use UV light irradiation on the cells, UV-related reactions such as lipid oxidation may affect repair of UV-induced DNA damage. However, this group's method is capable of avoiding such and limiting observation to removal of DNA damage by NER. Their probe will also possibly contribute to further studies of the DNA repair mechanism.
Abstract
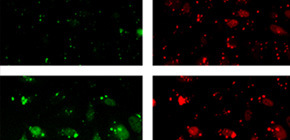
To maintain genetic integrity, ultraviolet light-induced photoproducts in DNA must be removed by the nucleotide excision repair (NER) pathway, which is initiated by damage recognition and dual incisions of the lesion-containing strand. We intended to detect the dual-incision step of cellular NER, by using a fluorescent probe. A 140-base pair linear duplex containing the (6–4) photoproduct and a fluorophore–quencher pair was prepared first. However, this type of DNA was found to be degraded rapidly by nucleases in cells. Next, a plasmid was used as a scaffold. In this case, the fluorophore and the quencher were attached to the same strand, and we expected that the dual-incision product containing them would be degraded in cells. At 3 h after transfection of HeLa cells with the plasmid-type probes, fluorescence emission was detected at the nuclei by fluorescence microscopy only when the probe contained the (6–4) photoproduct, and the results were confirmed by flow cytometry. Finally, XPA fibroblasts and the same cells expressing the XPA gene were transfected with the photoproduct-containing probe. Although the transfer of the probe into the cells was slow, fluorescence was detected depending on the NER ability of the cells.
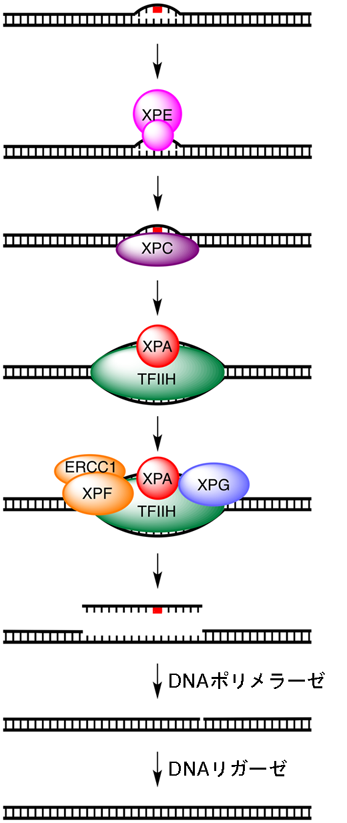
Figure 1
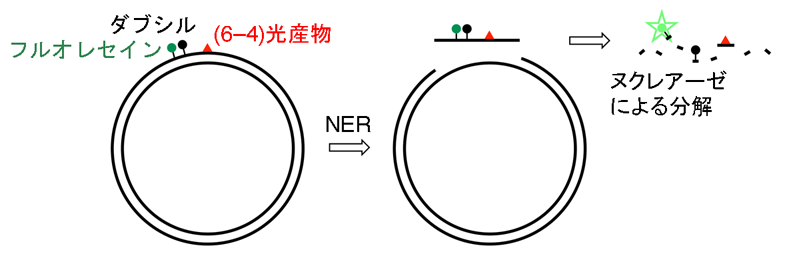
Figure 2

Figure 3
To learn more about this research, please view the full research report entitled " Fluorescence detection of cellular nucleotide excision repair of damaged DNA " at this page of the Scientific Reports website.
Related link :
