New cause of DNA damage verified
This type of DNA damage is thought to occur frequently
A research group under the leadership of KURAOKA Isao (Associate Professor, Graduate School of Engineering Science, Osaka University) has verified that oxidative damage by carboxymethyl cytosine occurring in the cytosine base as a new epigenetic cause of DNA damage. It is possible that this damage induces mutation and cell death, aging, carcinogenesis, etc. and, thus, is a new cause of diseases. This is considered to be something showing a new risk from DNA oxidation in humans and the importance of DNA repair.
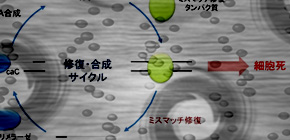
The group's results of the analysis of the DNA synthesis reaction in the cytosine base show that carboxymethyl cytosine inhibits the DNA synthesis reaction, moreover that through the DNA mismatched repair pathway, inhibition of the DNA synthesis reaction can lead to cell death.
The significance of these research results -- the importance of these results for society
DNA damage, leading to cell mutation and death, is a cause of many illnesses. The type of DNA damage which this group discovered is thought to occur frequently in response to epigenetic changes. Moreover, it is possible that this damage differs, as a path, from the TET family oxidase and is caused by oxidative stress. It is not yet clear what illnesses may be related to this damage; nevertheless, this group believes this damage shows a new danger from DNA oxidation and the importance of DNA repair.
Abstract
The genetic information encoded in genomes must be faithfully replicated and transmitted to daughter cells. The recent discovery of consecutive DNA conversions by TET family proteins of 5-methylcytosine into 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine (5caC) suggests these modified cytosines act as DNA lesions, which could threaten genome integrity. Here, we have shown that although 5caC pairs with guanine during DNA replication in vitro, G·5caC pairs stimulated DNA polymerase exonuclease activity and were recognized by the mismatch repair (MMR) proteins. Knockdown of thymine DNA glycosylase increased 5caC in genome, affected cell proliferation via MMR, indicating MMR is a novel reader for 5caC. These results suggest the epigenetic modification products of 5caC behave as DNA lesions.
To learn more about this research, please read the full research report entitled " Guanine- 5-carboxylcytosine base pairs mimic mismatches during DNA replication " at this page of the Scientific Reports website.
To learn more about this research, please read the full research report entitled " OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice " at this page of the PNAS website.
Related link :
