Mechanism for alleviating gout inflammation clarified
Relationship between lifestyle-related diseases & innate immune system activation
Under the leadership of SAITOH Tatsuya , Associate Professor, and AKIRA Shizuo , Professor and Director, Immunology Frontier Research Center ( IFReC ), Osaka University, a group of researchers conducting research on NLRP3 inflammasome, a component of the innate immune system, clarified a causative relationship between uric acid crystals and gout.
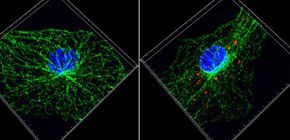
They found that accumulated uric acid crystals induced by hypernutrition harm mitochondria by strongly stimulating macrophages in the innate immune system. Harmed, the mitochondria decrease activity of SIRT2, an enzyme controlling microtubular function and a member of SIRT family related to health and longevity. The decrease in SIRT2 causes spatial mitochondria arrangements through microtubules, strongly promoting activation of NLRP3 inflammasome from the mitochondria that have been harmed.
Colchicine, a drug for treating gout, is thought to alleviate inflammation developed via NLRP3 inflammasome by suppressing the microtubule-driven spatial arrangement of mitochondria. This group clarified the mechanism by which uric acid crystals cause gout by identifying a new activation path of NLRP3 inflammasome.
The discovery of the importance of microtubles in NLRP3 inflammasome activation demonstrates that colchicine, a drug for treating gout, reduces symptoms of gout by suppressing the generation of inflammatory cytokines. However, colchicine also inhibits all functions of microtubules and, thereby, harms normal cells and tissues, an undesirable side effect. Therefore, its use as a therapeutic agent has decreased. A -tubulin acetyl transferase MEC17 was identified by these researchers as a factor for promoting NLRP3 inflammasome activation and is an enzyme for controling specific microtubular functions. Therefore, MEC17 is thought to be an ideal target drug to be developed as a therapeutic agent with fewer adverse side effects and as an alternative to colchicine. NLRP3 inflammasome is known to be related to the development of type 2 diabetes and arteriosclerosis. MEC17 is expected to become a target drug for diseases associated with adult lifestyle habits.
The SIRT family, homologs of Sir2 promoting longevity of yeast cells, has been found to be related to longevity in mammals, a topic that has attracted a lot of attention. This group also found, in examining SIRT2, that the SIRT family plays an important role in health maintenance by controlling inflammation.
Abstract
NLRP3 forms an inflammasome with its adaptor ASC, and its excessive activation can cause inflammatory diseases. However, little is known about the mechanisms that control assembly of the inflammasome complex. Here we show that microtubules mediated assembly of the NLRP3 inflammasome. Inducers of the NLRP3 inflammasome caused aberrant mitochondrial homeostasis to diminish the concentration of the coenzyme NAD+, which in turn inactivated the NAD+-dependent α-tubulin deacetylase sirtuin 2; this resulted in the accumulation of acetylated α-tubulin. Acetylated α-tubulin mediated the dynein-dependent transport of mitochondria and subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum. Therefore, in addition to direct activation of NLRP3, the creation of optimal sites for signal transduction by microtubules is required for activation of the entire NLRP3 inflammasome.




To learn more about this research, please read the full research report entitled " Microtubule-driven spatial mitochondria arrangement promotes NLRP3-inflammasome activation " at this page of the Nature Immunology website.
Related link :
