Detecting metallic lithium deposited on a battery anode using muonic X-rays
A group of researchers from The Institute of Materials Structure Science (IMSS) of the High Energy Accelerator Research Organization (KEK), Toyota Central R&D Labs., Inc., Osaka University, International Christian University, and the Comprehensive Research Organization for Science and Society (CROSS) has detected metallic lithium (Li) deposited on a battery anode at the decay muon beamline D2 in MUSE (MLF, J-PARC).
MLF: Materials and Life Science Experimental Facility
MUSE: Muon Science Laboratory
J-PARC: Japan Proton Accelerator Research Complex
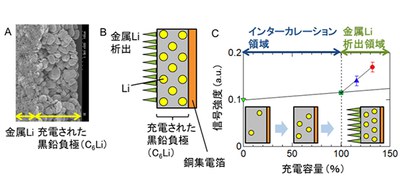
For recycling spent lithium-ion batteries (LiBs), the detection of the internal state of these batteries by nondestructive testing is of great importance. Under certain conditions, Li ions are reduced to metallic Li and form a dendrite, a treelike Li crystal growing on the anode surface, causing electrical shorts, overheating, and fires. Since metallic Li deposited on an electrode will not easily return to Li ions, it is important to detect Li dendrite formation in recycling spent LiBs.
X-ray fluorescence (XRF) spectroscopy is a non-destructive analytical technique used to determine the elemental composition of materials. Each of the elements in a sample produces a set of characteristic fluorescent X-rays that is unique for that specific element, which allows qualitative and quantitative analysis of material composition. However, it was difficult to detect X-rays emitted from Li through a container due to lithium’s low fluorescence yield using the XRF technique.
Thus, this joint group focused on muonic X-rays from Li. Muonic atoms can be easily formed by stopping negative muons inside a material. When a negative muon is captured in the irradiated material, a “muonic atom” is formed. As a negative muon has 200-times higher binding energy than a normal atom, muonic X-rays from any element can be detected with high sensitivity without being absorbed by the sample itself.
KEK developed a nondestructive high-sensitivity detection system to perform the elemental analysis of LiBs using muonic X-rays. Since high-intensity negative muonic beams of low energy were necessary for measuring samples with multi-layered electrode structure with a thickness of dozens of µm like Li-ion batteries, this group advanced research, producing decay muon beamline D2 in MUSE (MLF, J-PARC).
The researchers detected metallic Li deposited on the surface of an anode at this experimental facility -- a world first. In addition, using the difference in the atomic Coulomb capture ratio of the negative muons between the Li metal and ions, this group selectively detected Li metal. (The intensity of the muonic X-rays from metallic Li was higher than that from Li ions.)
In this study, the researchers removed the anode from the battery and packed it in an Al-laminated plastic sheet to analyze. Although it is difficult to analyze a Li-ion battery in an iron container with a thickness of 0.1mm or more in principle, it will be possible to detect metallic Li in Li-ion pouch cells.
This group’s achievements will expand the possibility of nondestructive detection of metallic Li in Li-ion batteries, increasing the safety of Li-ion batteries.
Figure 1
Figure 2
The article, “Nondestructive High-Sensitivity Detections of Metallic Lithium Deposited on a Battery Anode Using Muonic X-rays,” was published in Analytical Chemistry at DOI: https://doi.org/10.1021/acs.analchem.0c00370.
Related Links
Shinohara Laboratory, Graduate School of Science, Osaka University (link in Japanese)
