Structure and formation mechanism of type V pili of Porphyromonas gingivalis, clarified
A group of researchers from Osaka University, Okinawa Institute of Science and Technology Graduate University (OIST), and Nagasaki University has clarified the structure and assembly mechanism of the polymerized pilus of Porphyromonas gingivalis (P. gingivalis), one species of bacteria that causes periodontitis.
Periodontitis causes tooth loss and is also a potential risk factor for various diseases, such as diabetes and cardiovascular events. The cause of periodontitis is biofilm, commonly called "plaque," which adheres to tooth surfaces by P. gingivalis using its pili. Since pili serve as an adherence factor in colonization with host cells and biofilm formation, if biofilm formation is prevented, periodontitis can be prevented.
P. gingivalis has Type V pili, which differs from other pili types and is a key factor for its virulence. Members of the class Bacteroidia are commonly found in human gut microbiota and its importance in human health and aging has recently been emphasized. It is also pointed out that type V pili may be involved in the development of gut microbiota.
Pili are composed of anchor, stalk, accessory (or adaptor) and tip pilins, but the structure of type V pili and their assembly mechanisms were not known. Type V pili of P. gingivalis are made up of smaller protein units, called FimA pilins.
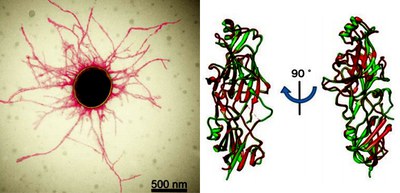
Researchers from Osaka University examined the structures of FimA pilins, clarifying differences in their structures at an atomic level by cryo-electron microscopy and crystallography.
Researchers from Nagasaki University and OIST showed that recombinant type V pilins could polymerize in vitro: recombinant FimA (rFimA) monomers self-assembled into long filaments after in vitro treatment with protease.
By combining analysis results using mutants as well, the researchers unveiled the mechanisms for the formation of type V pili of P. gingivalis, clarifying that, as long as an ample supply of stalk-pilin subunits exists on the cell membrane, pili can continue growing.
The formation of type V pili of P. gingivalis initiates with the release of donor strand of FimA pilin by N-terminal cleavage by protease: type V pili are assembled by a protease-mediated strand-exchange mechanism. The released donor strand in the donor-strand exchanged conformation was inserted into a groove of the adjacent FimA pilin, binding two pilins. In this way, type V pili assemble via a sequential polar assembly mechanism at the cell surface. Sequential addition of stalk pilins extends the growing pilus.
Findings in this study are a big step toward developing new drugs that target pili for diseases caused by gingivalis, such as periodontitis. These results will make a great contribution to the understanding of the development of gut microbiota, which plays an important role in human health.
Figure 1
Figure 2
FIgure 3
Figure 4
The article, “Structure of polymerized Type Vpilin reveals assembly mechanism involving protease-mediated strand-exchange,” was published in Nature Microbiology at DOI: https://www.nature.com/articles/s41564-020-0705-1
Related Links
Laboratory of Macromolecular Structure, Department of Macromolecular Science, Graduate School of Science, Osaka University (link in Japanese)
