Innovative whole-organ cell profiling method advances biological/medical research
A group of researchers from RIKEN and Osaka University Hospital developed the moving observation with efficient real-time autofocus (MOVIE) system, analyzing 103 million cells of a mouse brain. This method is suitable for clear, unobstructed brain imaging cocktails and computational analysis (CUBIC) procedures, a histology method that allows tissues to be transparent (a process called “tissue clearing”).
MOVIE is a high-speed imaging technology for efficient volumetric imaging, which integrates (i) continuous acquisition (MOVIE-scan), (ii) real-time autofocusing (MOVIE-focus), and (iii) skipping of blank images (MOVIE-skip).
When scanning samples in the z direction (depth), conventional microscopes use a ‘stop and exposure’ scheme. Since exposure is performed after moving to the specified position, this scheme takes a long time. In MOVIE-scan, the combination of uniform motion of the stage with synchronized continuous acquisition reduces the imaging time.
To obtain images of large samples with a microscope, one needs to subdivide the region of interest into multiple smaller images (referred to as tiles). In this tiling method, images of regions outside a sample are also acquired. In MOVIE-skip, pre-scan is performed when returning to the start position of the next stack of images to identify the start and end positions and search the edge of the sample and eliminate time to acquire images of regions outside the sample.
In MOVIE-focus, the light-sheet position is oscillated with a small step size as the image scan proceeds. The image quality metrics of two images before and after the light-sheet position change are evaluated in real-time and the focus value is updated. This real-time autofocus sequence enables high-speed and high-quality 3D imaging.
Using these technologies, this group created a customized light-sheet fluorescence microscope (LSFM). In LSFMs, a dichroic mirror is placed behind an objective lens for dual-color imaging to split the emission signals into two optical paths, enabling to focus these paths onto two scientific complementary metal–oxide–semiconductor (sCMOS) cameras. This allows for rapid and accurate acquisition of information about the relationship between the cell state and the cell spatial location, enabling the imaging of the transparent whole mouse brain within 5 to 12 hours.
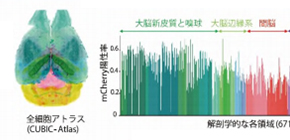
The group also proposed a cell-nucleus-detection algorithm based on 3D Hessian-based difference of Gaussian to implement a highly parallelized algorithm to utilize the parallel processing capabilities of multiple CPUs and graphics processing units (GPUs), analyzing the expanded CUBIC-X-treated mouse brain within 8 hours. They applied this analysis method to samples cleared by immersion in CUBIC agents, observing 103 million cells and updating the mouse brain atlas (CUBIC-Atlas). Furthermore, they administered adeno-associated virus (AAV)-PHP.eB vectors, AAV vectors encoding fluorescent proteins, to a mouse for whole mouse brain analysis, observing GFP expression in the cerebral cortex and olfactory areas.
This group’s achievements will be applied to fundamental research in the fields of biology and medicine, contributing to pathological diagnosis, drug discovery, and clinical pathological diagnosis, as a next-generation research base.
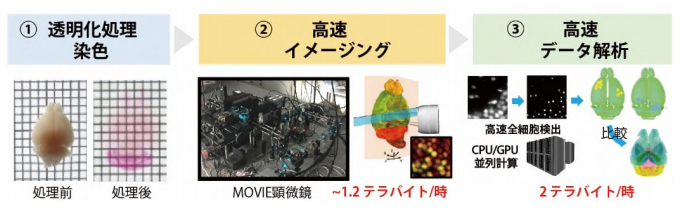
Figure 1

Figure 2

Figure 3

Figure 4

Figure 5
The article, “Advanced CUBIC tissue clearing for whole-organ cell profiling,” was published in Nature Protocols at DOI: http://dx.doi.org/10.1038/s41596-019-0240-9 .
Related Links
