Doctor-initiated clinical trial begun for cell spray heart failure treatment method
A team of researchers led by Professor SAWA Yoshiki of Osaka University Hospital developed a method of spraying mesenchymal stem cells onto the heart, commencing a doctor-initiated Phase 1 trial of a product that uses this spraying method, ADR-002K, in November 2019 at Osaka University Hospital. Details of this technique are published in Transplantation .
Statistics from 2017 show that heart disease is the second leading cause of death for Japanese people after malignant neoplasms. The prognosis of serious heart failure with ischemic cardiomyopathy (ICM) is particularly poor, but definitive therapy has not been established.
In order to treat serious heart failure, stem cell administration through coronary artery catheterization and intramyocardial injection of stem cells using a needle were performed, but there were concerns regarding the adverse effects and effectiveness of these medications.
In order to overcome these issues, a treatment method that involves skeletal myoblast cell sheet transplantation was developed and its effectiveness was proven to a certain extent; however, because it required the use of a cell processing center (CPC), it was difficult to put into practice at medical institutes without a CPC.
In a joint research project with ROHTO Pharmaceutical Co., using high-quality human allogeneic adipose-derived mesenchymal stem cells (ADSCs), this team developed a simple and convenient cell administration technique, which can be prepared in an operating room and available at low cost. Since this regenerative medicine technique does not require a CPC, it will be widely used at medical institutions around the country.
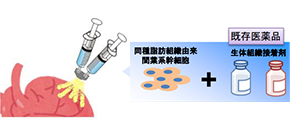
ADR-002K is a cell spray method-based product and is composed of ADR-002 as its main component and a biological adhesive solution as its sub-component. This clinical trial is being conducted in order to confirm the safety and feasibility of ADR-002K methodology for patients with ischemic cardiomyopathy who undergo coronary artery bypass surgery.
The widespread use of this method will promote the recovery of cardiac function in many ICM patients and improve their prognosis.
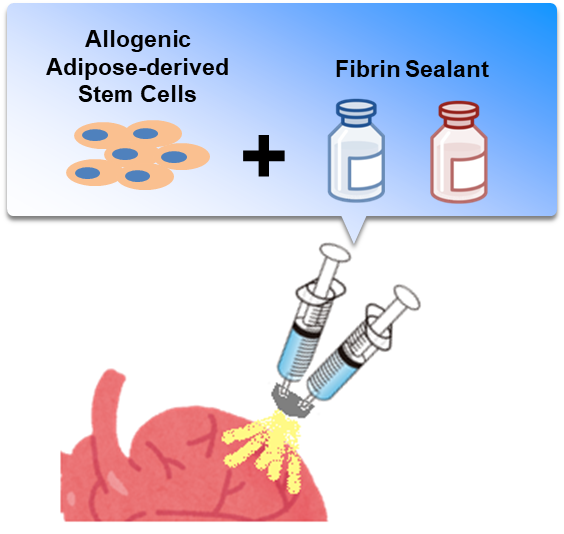
Figure: ADSCs are spread over the surface of the heart via cell spray in fibrinogen and thrombin solutions
The article, “Cell spray transplantation of adipose-derived mesenchymal stem cell recovers ischemic cardiomyopathy in a porcine model,” was published in Transplantation at DOI: https://doi.org/10.1097/TP.0000000000002385 .
Related links
