Mechanisms of respiratory enzyme activity in mitochondrial membrane unveiled
A group of researchers from the University of Hyogo and Osaka University unveiled a mechanism behind the activity of cytochrome c oxidase (CcO), a membrane-bound terminal enzyme in the mitochondrial electron transport chain (ETC), based on its molecular structures.
CcO reduces molecular oxygen to form water, allowing adenosine triphosphate (ATP) production. In the mitochondrial membrane, CcO exists in its monomeric form and in the form of a supercomplex (or a monomer bound to a complex).
In the membrane of a mitochondria, or the “engine” of the cell, electrons are transferred to complex I, complex III, and then complex IV (CcO) through the ETC. In CcO, four electrons are removed from four molecules of cytochrome c and are transferred to molecular oxygen (O 2 ), producing two molecules of water (H 2 O).
Energy released by the electron transfer processes pumps protons (hydrogen ions) to the intermembrane region, where they accumulate in a high enough concentration to phosphorylate the adenosine diphosphate (ADP) to ATP.
Recent structural analyses of the mitochondrial respiratory supercomplex revealed that CcO exists in its monomeric form in the respiratory supercomplex, namely, a CcO monomer associates with a complex I and a complex III. However, since it was not possible to strictly compare the activity of monomeric and dimeric enzymes in the past, physiological significance of why CcO takes different forms was unknown.
In this study, the group of researchers stabilized CcO in the monomeric state and the dimeric state, respectively, to compare their enzyme activity and molecular structures and found:
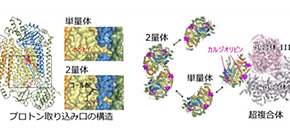
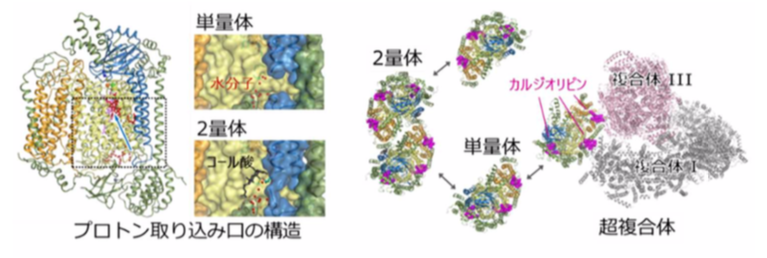
・In dimetric CcO, its structure is stabilized because of cholate (CH 2 ) bonding. It is thought that the affinity and release of CH 2 is responsible for the differences in the activity of monomeric and dimeric CcO, the active and standby forms of CcO.
・Structural comparison revealed that a hydrogen bond network of water molecules was formed at the entry surface of the proton transfer pathway (termed the K-pathway) in monomeric CcO. The structural alteration at the entry point of the K-pathway caused by the release of CH 2 promotes uptake of protons, causing the activation of CcO by monomerization.
In addition, the crystal structure showed that cardiolipins, a kind of phospholipid bound to complex I and complex III, were also involved in the formation of a supercomplex. It is thought that the formation of supercomplexes allows for more efficient electron transport (necessary of oxygen reduction) and affects the stability of CcO.
This group’s research results suggest that CcO in the monomeric state, dimeric state, and supercomplex state, depending on cardiolipins, are involved in regulation of energy generation via the respiratory electron transport chain.
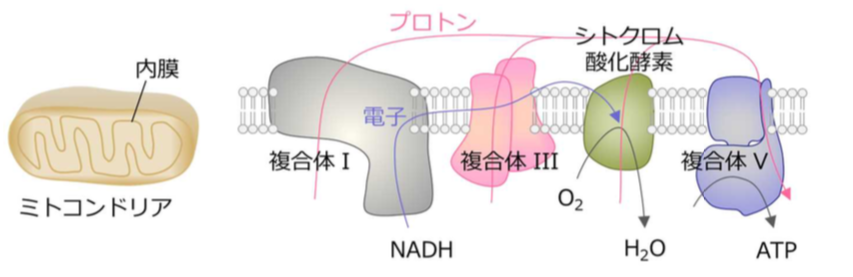
Figure 1
Figure 2
The article, “Monomeric structure of an active form of bovine cytochrome c oxidase,” was published in Communications Biology at DOI: https://www.pnas.org/content/116/40/19945 .
Related Links
