Nitric oxide synthesis induced by parasite infection subverts host immune responses
Mechanism of human immune suppression by toxoplasma pathogenicity factor GRA15 elucidated
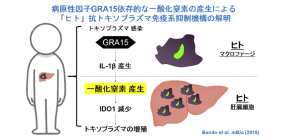
A group of researchers led by Professor Masahiro YAMAMOTO at the Research Institute for Microbial Diseases, Osaka University clarified that toxoplasma pathogenicity factor GRA15 activated host immune responses to produce nitric oxide (NO) and suppressed anti- T. gondii cell-autonomous immune responses.
Toxoplasma gondii (T. gondii) is a parasite that can only multiply within host cells they have invaded and releases various effector molecules such as GRA15 into the cells, activating the host's immune systems. Research using mice showed that GRA15-deficient T. gondii was more virulent than wild-type (WT) T. gondii . This means that lack of GRA15 in T. gondii promotes its proliferation in host cells. It should be advantageous for T. gondii to be without GRA15, but since GRA15 is found in T. gondii , the role of GRA15 in T. gondii was unknown.
T. gondii -infected macrophages travel throughout various organs in the host body, so the group performed tests in a coculture of hepatocytes and T. gondii -infected macrophages. Since T. gondii -infected macrophages produce and release cytokines such as interleukin (IL1) in a manner dependent on GRA15, GRA15-deficient T. gondii cannot proliferate in the presence of interferon. So, the researchers directly activated IL-1 on hepatocytes.
In combination with interferon, IL-1 stimulated the expression of inducible Nitric Oxide Synthase (iNOS), an enzyme to synthesize NO in hepatocytes, inducing the synthesis of NO. This dramatically reduced the levels of indole 2,3-dioxygenase 1 (IDO1), a critically important IFN-γ-inducible anti-Toxoplasma protein in humans, suppressing immune responses.
From these results, it was found that T. gondii suppressed the IFN-γ-induced anti- T. gondii responses in a manner dependent on GRA15. It was thought that inducible nitric oxide synthase (iNOS) was an anti-Toxoplasma host factor in mice, but contrary to that, in humans, iNOS actually suppressed anti- T. gondii responses. In addition, the researchers confirmed that iNOS inhibitor suppressed the proliferation of wild-type (WT) T. gondii that had GRA15, in cocultures of THP-1 and Huh7 cells as well.
These results revealed the critical role of NO in the suppression of anti- T. gondii responses in humans. It is anticipated that blocking of NO production will become a novel therapeutic approach for treating human toxoplasmosis.
Abstract
Although Toxoplasma virulence mechanisms targeting gamma interferon (IFN-γ)-induced cell-autonomous antiparasitic immunity have been extensively characterized in mice, the virulence mechanisms in humans remain uncertain, partly because cell-autonomous immune responses against Toxoplasma differ markedly between mice and humans. Despite the identification of inducible nitric oxide synthase (iNOS) as an anti-Toxoplasma host factor in mice, here we show that iNOS in humans is a pro-Toxoplasma host factor that promotes the growth of the parasite. The GRA15 Toxoplasma effector-dependent disarmament of IFN-γ-induced parasite growth inhibition was evident when parasite-infected monocytes were cocultured with hepatocytes. Interleukin-1β (IL-1β), produced from monocytes in a manner dependent on GRA15 and the host’s NLRP3 inflammasome, combined with IFN-γ to strongly stimulate iNOS expression in hepatocytes; this dramatically reduced the levels of indole 2,3-dioxygenase 1 (IDO1), a critically important IFN-γ-inducible anti-Toxoplasma protein in humans, thus allowing parasite growth. Taking the data together, Toxoplasma utilizes human iNOS to antagonize IFN-γ-induced IDO1-mediated cell-autonomous immunity via its GRA15 virulence factor.
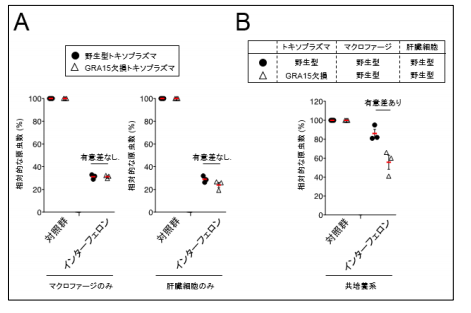
Figure 1
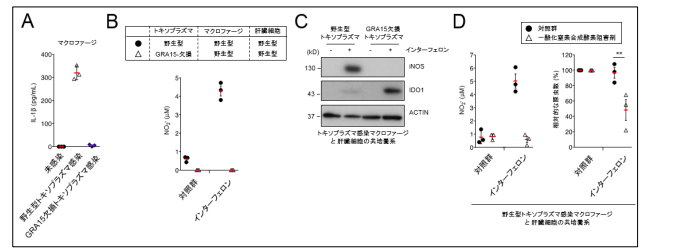
Figure 2
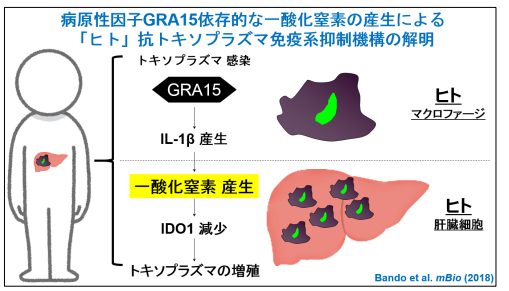
Figure 3
The article “Inducible Nitric Oxide Synthase Is a Key Host Factor for Toxoplasma GRA15-Dependent Disruption ofthe Gamma Interferon-Induced Antiparasitic Human Response” was published in mBio at DOI: https://doi.org/10.1128/mBio.01738-18.
Related links
- Research Institute for Microbial Diseases (link in Japanese)
