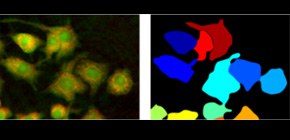
Multiple optical measurements reveal the single cell activation without contrast agent
Points
- Label-free optical measurements can study fine cellular changes such as macrophage cells activation.
- Single-cell indicators demonstrate the heterogeneity of cellular responses both in morphology and molecular content.
- Morphology (phenotype) and molecular content are indicative of different phenomena in the activation cascade.
- Selective inhibition of certain pathways in the activation cascade can also be observed through label-free indicators, showing selective molecular expression in the case of partial activation.
Summary
Nicolas Pavillon (Assistant Professor), Nicholas I. Smith (Associate Professor, Immunology Frontier Research Center, Osaka University) and collaborators developed a label-free multimodal microscopy platform that allows the non-invasive study of cellular preparations without the need of any additional chemicals or contrast agent. The parameters extracted from these measurements, coupled with machine algorithms, enable the study of fine cellular processes such as macrophage cells activation upon exposure to lipopolysaccharide (LPS). The authors demonstrate that activation, as well as partial activation inhibition, can be observed at single-cell level through phenotypic and molecular characterization purely through non-invasive optical means.
Background
Label-free optical measurements have become popular thanks to their ability to observe samples non-invasively. Techniques such as quantitative phase microscopy or Raman spectroscopy, as used in this study, have been extensively used in recent years to characterize specimens and identify cells from different origins. However, the specificity of these approaches usually only allows for the discrimination of cell types. The group shows in this study that fine processes such as macrophage activation within populations of identical cells is also possible.
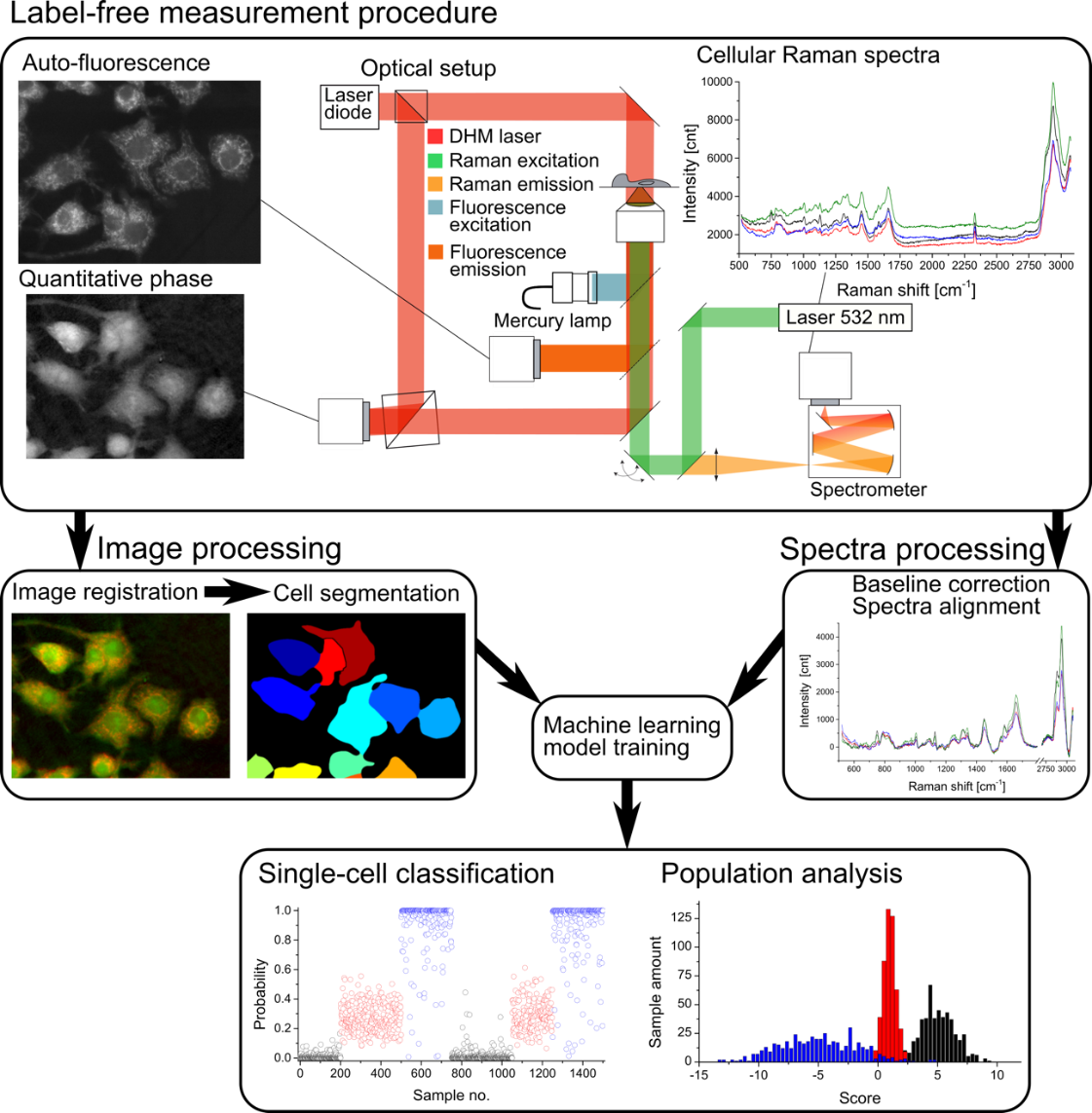
Figure: Measurement and data treatment principle, where morphological and spectral parameters are employed to generate a statistical model that allows for analysis of new cellular data taken in later experiments for classification and analysis.
Significance of the findings
Standard analysis methods are often destructive to the sample, or rely on contrast agents to detect specific molecules of interest, which limits the study to known target molecules. The approach developed here is based on non-invasive techniques that rely on the endogenous contrast of the sample, i.e. on its phenotype and whole intracellular molecular content. Furthermore, standard assays, despite their sensitiveness, often measure samples at a population level, taking advantage of the cumulative effect on secreted molecules, but losing the information about individual cellular response. The presented approach provides measurements at single-cell level, enabling the study of cell-to-cell variability within populations.
Technical terms
- Raman spectroscopy: An optical method that measures the vibrational modes of molecules, enabling their identification through spectral features. Applied to living cells, it is an indirect non-invasive method to assess their molecular content.
- Quantitative phase microscopy: An optical imaging method that enables dynamic measurement of live cell cultures, providing quantitative information about the local optical density of cells, related to the refractive index.
Article information
Journal: Proceedings of the National Academy of Science of the USA; PNAS (Online publishing on March 5, 2018)
Title: “Noninvasive detection of macrophage activation with single-cell resolution through machine learning”
Authors: Nicolas Pavillon, Alison J. Hobro, Shizuo Akira, and Nicholas I. Smith.
To learn more about this research, please view the full research report entitled " Noninvasive detection of macrophage activation with single-cell resolution through machine learning " at this page of Proceedings of the National Academy of Science of the USA .
Related links
