Peptide for inhibiting the function pf protein that may cause cancer infiltration and osteoporosis created
Will become an active region in designing new drugs
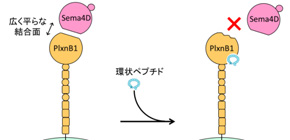
Semaphorin is a protein that is responsible for neurogenesis, organogenesis, and immune responses, and plexins comprise membrane proteins and act as receptors for transducing semaphorin signals. Multiple ligand-receptor pairs are created between two families, semaphorin and plexin, and Sema4D-PlxnB1 signaling in particular is known to promote cancer infiltration and reduced bone mass. Therefore, it is thought that inhibiting this signal transduction pathway will be helpful in treating cancer and osteoporosis. However, as the binding surface of Sema4D to PlxnB1 was large and flat and had no indents in which low-molecule chemical compounds could get stuck, it was difficult to design chemical compounds to inhibit binding between the two.
A group of researchers led by Professor TAKAGI Jun'ichi, Institute for Protein Research, Osaka University, together with a group of researchers led by Professor SUGA Hiroaki, Graduate School of Science, The University of Tokyo, succeeded in obtaining plexin B1 (PlxnB1)-binding macrocyclic peptide, PB1m6. PlxnB1 is a semaphorin 4D (Sema4D) receptor, and this group also proved that PB1m6 inhibited the function of PlxnB1.
PlxnB1 is a membrane protein that causes signal transduction by binding to Sema4D and is involved in various diseases, such as cancer and osteoporosis. Macrocyclic peptide PB1m6 obtained in this study weakened the binding force between Sema4D and PlxnB1 by binding to the region distant from the Sema4D-binding site, completely inhibiting biological activity of Sema4D-PlxnB1. These effects are called “allosteric regulation” and PB1m6 is an allosteric inhibitor of PlxnB1. The binding site of PB1m6 on PlxnB1, or the allosteric site, will become a new target region for anti-semaphorin-plexin drug development and macrocyclic peptide PB1m6 will have a place in research and development of middle-molecule drugs.
Abstract
Semaphorin axonal guidance factors are multifunctional proteins that play important roles in immune response, cancer cell proliferation, and organogenesis, making semaphorins and their signaling receptor plexins important drug targets for various diseases. However, the large and flat binding surface of the semaphorin-plexin interaction interface is difficult to target by traditional small-molecule drugs. Here, we report the discovery of a high-affinity plexin B1 (PlxnB1)-binding macrocyclic peptide, PB1m6 (K D = 3.5 nM). PB1m6 specifically inhibited the binding of physiological ligand semaphorin 4D (Sema4D) in vitro and completely suppressed Sema4D-induced cell collapse. Structural studies revealed that PB1m6 binds at a groove between the fifth and sixth blades of the sema domain in PlxnB1 distant from the Sema4D-binding site, indicating the non-competitive and allosteric nature of the inhibitory activity. The discovery of this novel allosteric site can potentially be used to target plexin family proteins for the development of drugs that modulate semaphorin and plexin signaling.
Figure 1
Figure 2
To learn more about this research, please view the full research report entitled “ Allosteric inhibition of a semaphorin 4D receptor plexinB1 by a high-affinity macrocyclic peptide ” at this page of the Cell Chemical Biology website.
Related link
