Maintenance of ring-shaped proteins prevents mutations
A mechanism for repairing errors in DNA synthesis and preventing mutations clarified
A joint group of researchers led by Assistant Professor TAKAHASHI Tatsuro, Graduate Student KAWASOE Yoshitaka, Associate Professor NAKAGAWA Takuro, and Professor MASUKATA Hisao at the Graduate School of Science, Osaka University, and Professor TSURIMOTO Toshiki at the Graduate School of Science, Kyushu University, clarified a system for repairing errors in DNA synthesis and preventing mutations and cancer.
For cells to increase, it’s necessary to copy genetic information coded in DNA, but errors in the copying process occasionally occur. If these errors are left unrepaired, genetic changes, or mutations, occur. When mutations accumulate, the correct proteins are not produced, turning normal cells into cancer cells.
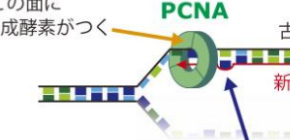
DNA mismatch repair is an important mechanism for correcting errors that happen during DNA replication. DNA is formed of two strands. In DNA replication, the parental strands separate, with each serving as a template so that new strands are made. The parental strands retain correct information, and errors are included in newly made strands. Mismatch repair differentiates the parental strand from the new one and repairs only the new strand with errors; however, the details of this response mechanism were not understood.
This group reconstructed in vitro mismatch repair by using eggs of African clawed frogs, experimentally proving that Proliferating Cell Nuclear Antigen (PCNA), which is necessary for DNA replication, had information for distinguishing the parental and daughter DNA strands. Furthermore, this group discovered that mismatch repair maintains strand information for an extended period by inhibiting unloading of PCNA from DNA.
While an accumulation of mutations causes cancer and genetic diseases, threatening people’s health, changes in genetic information are important in terms of normal immune response and as a driving force for evolution of living things. It is expected that this group’s achievement will be helpful in the clarification of the cause of mutation accumulation in humans, as well as in selective breeding by artificially accelerating evolution.
Abstract
Eukaryotic mismatch repair (MMR) utilizes single-strand breaks as signals to target the strand to be repaired. DNA-bound PCNA is also presumed to direct MMR. The MMR capability must be limited to a post-replicative temporal window during which the signals are available. However, both identity of the signal(s) involved in the retention of this temporal window and the mechanism that maintains the MMR capability after DNA synthesis remain unclear. Using Xenopus egg extracts, we discovered a mechanism that ensures long-term retention of the MMR capability. We show that DNA-bound PCNA induces strand-specific MMR in the absence of strand discontinuities. Strikingly, MutSα inhibited PCNA unloading through its PCNA-interacting motif, thereby extending significantly the temporal window permissive to strand-specific MMR. Our data identify DNA-bound PCNA as the signal that enables strand discrimination after the disappearance of strand discontinuities, and uncover a novel role of MutSα in the retention of the post-replicative MMR capability.
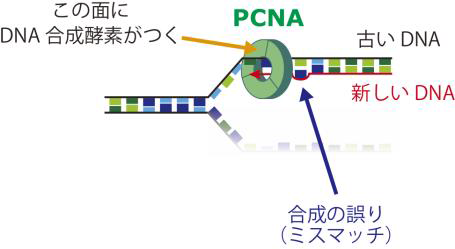
Figure 1
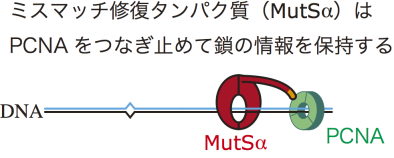
Figure 2
To learn more about this research, please view the full research report entitled “ MutSα maintains the mismatch repair capability by inhibiting PCNA unloading ” at this page of the eLife website.
Related link
