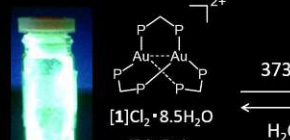
Successful synthesis of gold compounds showing reversibly emitting light in response to heating and grinding
Will be used for luminescent sensor devices and luminous paint
The international joint research group led by Professor Takumi KONNO (Graduate School of Science, Osaka University) discovered an extremely emissive gold(I) compound, which shows reversible, two-step emission color change in response to heating and grinding (Figure 1), and elucidated the conversion mechanism. In this compound, each of two gold(I) ions adopts a ‘3-coordinated trigonal-planar’ geometry, rather than a usual ‘2-coordinated linear’ geometry. Based on this finding, gold(I) chromic compounds, the emission colors of which are made to vary in respond to external stimuli, will be used to various applications, such as luminous paints and chemical sensors.
Abstract
Photoluminescent compounds showing emission color changes in response to external stimuli have received considerable attention because of their wide range of applications. In this work, the unique photoluminescence behavior of a digold(I) coordination system with trigonal-planar Au I centers, [Au 2 (dppm) 3 ] 2 + (dppm = bis(diphenylphosphino)methane), was reported. This system showed an extremely intense phosphorescence, with a quantum yield of > 95% in the solid state. Both the emission color and thermal stability varied due to changing counter ions (Cl − vs. OTf − ). Of particular note was the thermal crystalline-amorphous-crystalline transformation for the chloride salt, which was accompanied by drastic emission color changes. Single-crystal and powder X-ray diffractions demonstrated that the two-step transformation was induced by the loss of water molecules of crystallization with the subsequent removal of a dppm ligand to form [Au 2 (dppm) 2 ] 2 + , which was mechanically reverted back to [Au 2 (dppm) 3 ] 2 + .
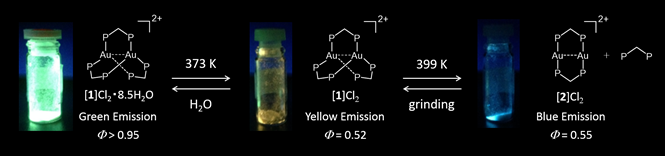
Figure 1 Reversible conversion of “Green emitting complex”, “yellow emitting complex”, and “blue emitting complex” by heating and by mechanical force.
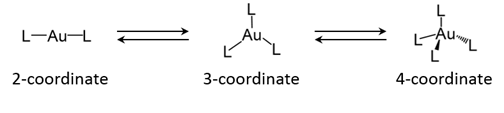
Figure 2 Typical coordination geometry of gold(I) complex: two-coordinated linear, three-coordinated trigonal-planar, and four-coordinated tetrahedral geometries(L: ligand)
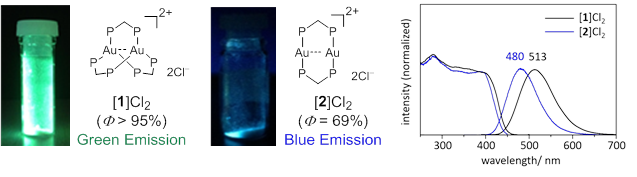
Figure 3 The pictures, structures (left), and emission spectra (right) of three-coordinated trigonal-planar [ 1 ]Cl 2 (green emission) and two-coordinated linear [ 2 ]Cl 2 (blue emission)
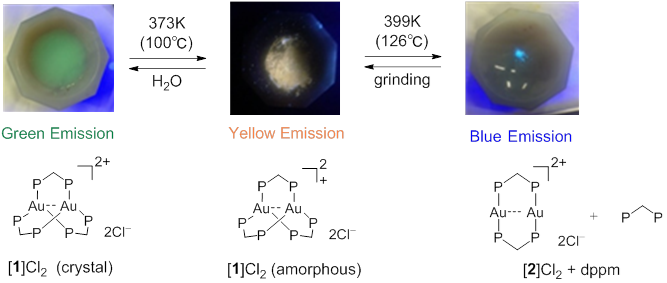
Figure 4 The structural (lower) and emission color changes (upper) of [ 1 ]Cl 2 by heating
To learn more about this research, please view the full research report entitled “ Crystalline-Amorphous-Crystalline Transformation in a Highly Brilliant Luminescent System with Trigonal-Planar Gold(l) Centers ” at this page of the Scientific Reports website.
Related link
