New light shed on the development of treatment for complications associated with Down syndrome
Mechanism behind complications clarified using human iPS cells and genome editing
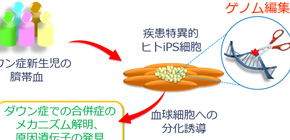
A group of researchers led by Professor OHZONO Keiichi and Assistant Professor KITABATAKE Yasuji (Department of Medical Informatics, Graduate School of Medicine, Osaka University) produced diverse types of cellular disease models of Down syndrome by using human induced pluripotent stem cells (iPSCs) and genome-editing technology.
This group clarified the pathological mechanism of Transient Abnormal Myelopoiesis (TAM), which often occurs in newborn babies with Down syndrome, identifying the responsible area on chromosome 21 and major TAM-causing-genes. Down syndrome is caused when an individual has three copies of chromosome 21 instead of the usual two copies. Because of this, unlike single-gene disorders, in Down syndrome, quantitative changes occur in more than 100 genes at the same time.
In order to understand the clinical condition, it’s important to know what combination of changes in 330 genes on chromosome 21 are related to it. However, this was very difficult because analyzing enormous amounts of combination was required.
By using genome editing in human iPS cells, this group identified the critical region of Down syndrome, which causes hematopoietic abnormalities, clarifying major gene groups in the region. By performing various genome editing on GATA1 genes using iPS cells produced from cord blood of healthy children and Down syndrome children with/without TAM, this group produced more than 40 strains of iPS cells of 20 different types, reproducing the clinical condition of TAM. As a result, it was found that GATA with trisomy 21 and genetic mutations have the following features:
1. Trisomy 21 facilitates the excessive proliferation of blood cells.
2. GATA1 mutations disturb the differentiation of blood, megakaryoblastic cells, in particular.
It was also found that gene groups, the amount of their expression drastically changed along with hematopoietic abnormalities, existed on chromosome 21 and that they were located in the limited region called 4-Mb. This group produced “partial trisomy iPS cells” in which the 4-Mb region on chromosome was removed. As expected, this partially trisomy iPS cells lost the feature of hematopoietic abnormalities, so it was found this 4-Mb region was responsible for hematopoietic abnormalities in Down syndrome.
It is expected that this group’s achievement will lead to the establishment of diagnosis and treatment of leukemia, which often occurs in Down syndrome. This group’s results present new methods for researching various clinical conditions associated with Down syndrome such as mental development disorder and abnormal cognitive function. From these things, this group’s research results will become a first step toward the development of Down syndrome research in the future.
Abstract
Chromosomal aneuploidy and specific gene mutations are recognized early hallmarks of many oncogenic processes. However, the net effect of these abnormalities has generally not been explored. We focused on transient myeloproliferative disorder (TMD) in Down syndrome, which is characteristically associated with somatic mutations in GATA1. To better understand functional interplay between trisomy 21 and GATA1 mutations in hematopoiesis, we constructed cellular disease models using human induced pluripotent stem cells (iPSCs) and genome-editing technologies. Comparative analysis of these engineered iPSCs demonstrated that trisomy 21 perturbed hematopoietic development through the enhanced production of early hematopoietic progenitors and the upregulation of mutated GATA1, resulting in the accelerated production of aberrantly differentiated cells. These effects were mediated by dosage alterations of RUNX1, ETS2, and ERG, which are located in a critical 4-Mb region of chromosome 21. Our study provides insight into the genetic synergy that contributes to multi-step leukemogenesis.
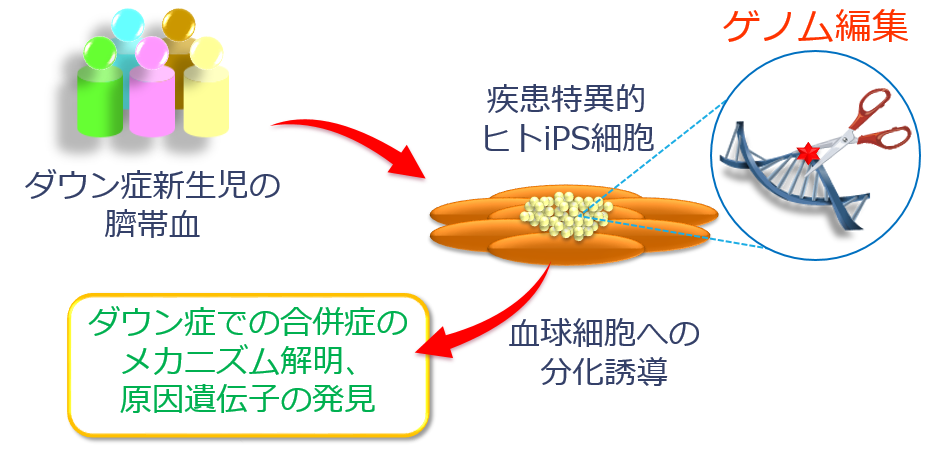
Figure 1
Figure 2
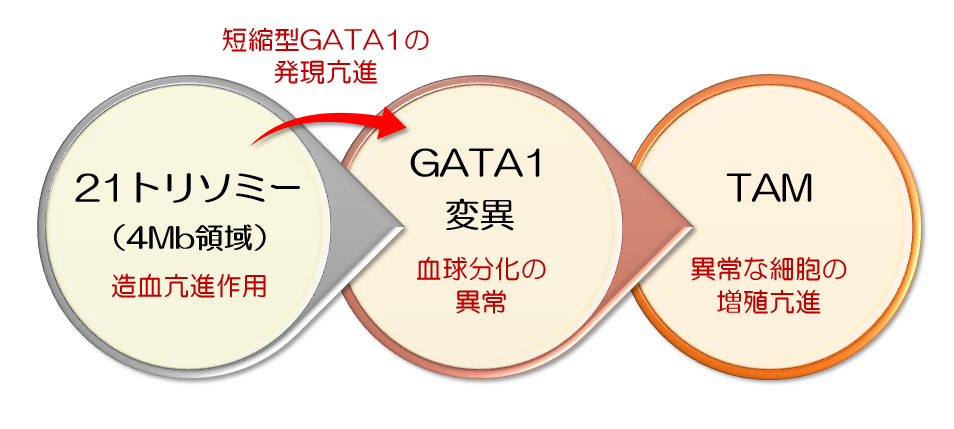
Figure 3
To learn more about this research, please view the full research report entitled " Systematic cellular disease models reveal synergistic interactions of trisomy 21 and GATA1 mutations in hematopoietic abnormalities " at this page of the Cell Reports website.
Related link
- HP Department of Pediatrics, Graduate School of Medicine, Osaka University (link in Japanese)
