Drugs to be available at moderate prices
Super-effective drug synthesis method established
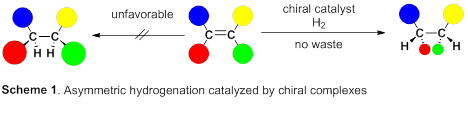
A group of researchers led by Professor MASHIMA Kazushi (Graduate School of Engineering Science, Osaka University) succeeded in synthesizing optically active organic compounds, the basic skeleton of drugs and functional materials. This reaction is one in which clean source hydrogen adds to carbon-carbon double bonds to form chiral carbon (asymmetric hydrogenation), which is drawing attention as a non-waste producing atom economy reaction.
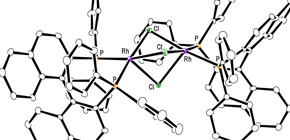
This group led by Prof. Mashima clarified that iridium binuclear complexes had shown high hydrogeneration of carbon-nitrogen double bonds and that the use of them made it possible to control the steric conformation of products. However, with iridium binuclear complexes, a sufficient reaction had not been available in asymmetric hydrogenation of simple olefin.
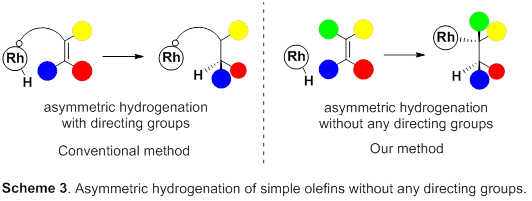
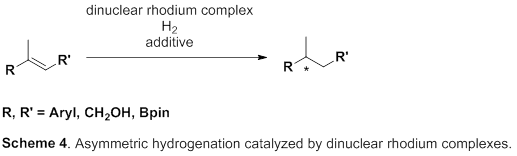
So, this group focused on rhodium metal, which belongs to group 9 of the periodic table, just like iridium metal. Asymmetric hydrogenation using rhodium metal has been long examined and has a high level of hydrogenation; however, for asymmetric hydrogenation of olefins, directing groups, which make it easy to control steric conformation, are needed on olefins. However, in order to introduce such directing groups, it’s necessary to change the skeleton of the targeted product, which is unfit for partial skeleton construction of drugs. Therefore, the development of excellent asymmetric hydrogenation of simple olefin without directing groups has been sought after.
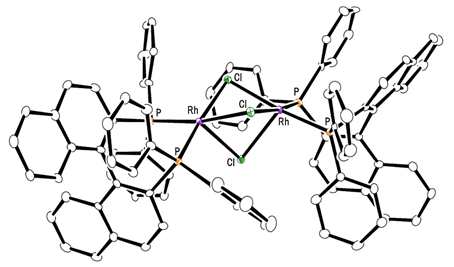
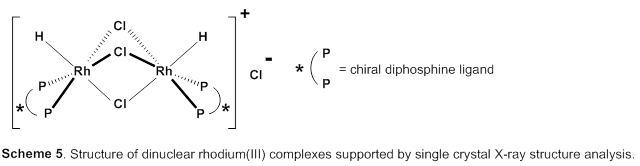
This group succeeded in synthesis of optically active rhodium binuclear complexes, which have the same structure as iridium binuclear complexes, achieving asymmetric hydrogenation of simple olefin by using rhodium binuclear complexes as catalysts. This group’s asymmetric hydrogenation using optically active rhodium binuclear complexes provides a new synthesis approach to obtaining chemical compounds with high optical purity, which has been difficult to achieve. It is expected that the development of this asymmetric reaction can be applied to the synthesis of various medicines, so it will make it possible to smoothly produce drugs whose synthesis has been difficult, leading to medicines being provided to society at low prices.
With the possibility of easily constructing optically active complex skeletons comes research regarding seed chemicals to drug discovery, and to the development of new drugs. Furthermore, this asymmetric reaction enabling acquisition of desired objects with high optical purity will be applied to ferroelectric liquid crystals, chiral functional materials that have drawn attention in recent years. This study will contribute to society in both drug development and industrial production.
Abstract
Efficient rhodium(III) catalysts were developed for asymmetric hydrogenation of simple olefins. A new series of chloride-bridged dinuclear rhodium(III) complexes was synthesized from a rhodium(I) precursor, [RhCl(cod)] 2 , chiral diphosphine ligands, and HCl. Dinuclear rhodium(III) complexes acted as efficient catalysts for asymmetric hydrogenation of (E)-prop-1-ene-1,2-diyldibenzene and its derivatives without any directing groups, in sharp contrast to widely-used rhodium(I) catalytic systems that require a directing group for high enantioselectivity. In addition, the present catalytic system was applied to asymmetric hydrogenation of allylic alcohols, alkenylboranes, and unsaturated cyclic sulfones. Controlled experiments supported the superiority of dinuclear rhodium(III) complexes over typical rhodium(I) catalytic systems.
To learn more about this research, please view the full research report entitled “ Chloride-Bridged Dinuclear Rhodium(III) Complexes Bearing Chiral Diphosphine Ligands: Catalyst Precursors for Asymmetric Hydrogenation of Simple Olefins ” at this page of the S cientific Reports website.
Related link
